Hemostasis Evaluation of Antibacterial and Highly Absorbent Composite Wound Dressings in Animal Hemostasis Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyacrylate/Tencel® (PT) Nonwoven Composites
2.3. Preparation of Nano Ag-Chitosan Composite Dressing
2.4. Tensile Strength Test
2.5. X-ray Diffractometer (XRD) Analysis
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Antibacterial Measurement
2.8. Ag Release Rate of PT Composite Dressing
2.9. SEM Surface Observation
2.10. Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT)
2.11. Animal Test
2.12. Statistical Analyses
3. Results and Discussion
3.1. Effects of Polyacrylate Fiber Content and Fiber Orientation on Tensile Strength of PT Nonwoven Composites
3.2. Effects of Polyacrylate Fiber Content and Fiber Orientation (MD/CD) on Vertical Wicking Length of PT Nonwoven Composites
3.3. FTIR Analyses of Chitosan/Ag Membranes
3.4. Effects of Reaction Time on UV-Vis Absorbent Spectra of Ag Nanoparticles
3.5. Effects of Chitosan Concentration and Reaction Time on X-ray Diffraction Spectrum of Chitosan/Ag Membranes
3.6. Water and Blood Absorption of PT Nonwoven Composites
3.7. Antibacterial Property of PT Nonwoven Composites
3.8. Ag Release Rate of PT Nonwoven Dressing
3.9. PT, APTT, and Platelet Agglutination Analyses of PT Nonwoven Composites
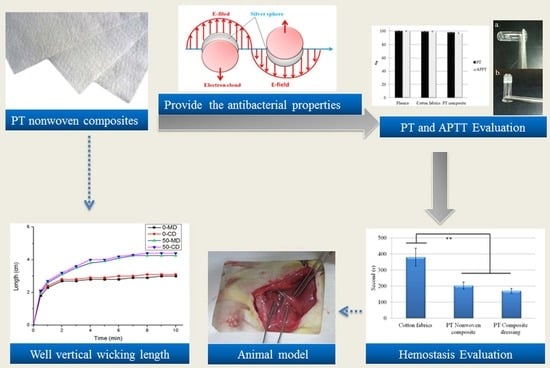
3.10. Hemostasis Evaluation of PT Nonwoven Composites in Animal Hemostasis Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, A.; Mahmood, B.; Majid, R.A.; Narendra, P.S.C.; Sapana, J.; Maryam, D.S.; Payam, Z. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar]
- Deng, C.M.; He, L.Z.; Zhao, M.; Yang, D.; Liu, Y. Biological properties of the chitosan-gelatin sponge wound dressing. Carbohydr. Polym. 2007, 69, 583–589. [Google Scholar] [CrossRef]
- Keith, F.C.; Richard, J.W. Criteria for identifying wound infection—Revisited. Ostomy Wound Manag. 2005, 51, 28–34. [Google Scholar]
- Hutchinson, R.W.; George, K.; Johns, D.; Craven, L.; Zhang, G.; Shnoda, P. Hemostatic efficacy and tissue reaction of oxidized regenerated cellulose hemostats. Cellulose 2013, 20, 537–545. [Google Scholar] [CrossRef]
- Wu, Y.D.; He, J.M.; Cheng, W.L.; Gu, H.B.; Guo, Z.H.; Gao, S.; Huang, Y.D. Oxidized regenerated cellulose-based hemostat with microscopically gradient structure. Carbohydr. Polym. 2012, 88, 1023–1032. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Yoon, H.S.; Kim, H.K.; Kim, K.S. Efficacy of Oxidized Regenerated Cellulose, SurgiGuard (R), in Porcine Surgery. Yonsei Med. J. 2017, 58, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.L.; Lv, L.; Bai, Y.J.; Yang, H.L.; Zhou, H.; Li, C.D.; Yang, L. Nanotechnology-enabled materials for hemostatic and anti-infection treatments in orthopedic surgery. Int. J. Nanomed. 2018, 13, 8325–8338. [Google Scholar] [CrossRef] [Green Version]
- Robatto, G.; Malinverno, G.; Bootman, J. Development and implementation of a safety evaluation program for chemical fibers. Regul. Toxicol. Pharmacol. 1993, 17, 193–208. [Google Scholar] [CrossRef]
- Bredereck, K.P.D.; Hermanutz, F.D. Man made cellulosics. Coloration Technol. 2008, 35, 59–75. [Google Scholar] [CrossRef]
- Huong Mai, B.; Ehrhardt, A.; Bechtold, T. CI Reactive Black 5 dye as a visible crosslinker to improve physical properties of lyocell fabrics. Cellulose 2009, 16, 27–35. [Google Scholar] [CrossRef]
- Chen, J.H.; Wang, K.; Xu, F.; Sun, R.C. Effect of hemicellulose removal on the structural and mechanical properties of regenerated fibers from bamboo. Cellulose 2015, 22, 63–72. [Google Scholar] [CrossRef]
- Wang, P.; Tawiah, B.; Tian, A.L.; Wang, C.X.; Zhang, L.P.; Fu, S.H. Properties of alginate fiber spun-dyed with fluorescent pigment dispersion. Carbohydr. Polym. 2015, 118, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Schuster, K.C.; Suchomel, F.; Männer, J.; Abu-Rous, M.; Firgo, H. Functional and comfort properties of textiles from Tencel® fibres resulting from the fibres’ water-absorbing nanostructure: A review. Macromol. Symp. 2006, 244, 149–165. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Seiichi, O.; Kento, M.; Arvind, K.S.C.; Pan, Q.; Noriko, N.; Akiko, N.; Hiromi, Y.; Gento, Y.; Yuichi, H.; Ryo, S.; et al. Silver-loaded carboxymethyl cellulose nonwoven sheet with controlled counterions for infected wound healing. Carbohydr. Polym. 2022, 286, 119289. [Google Scholar]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef]
- He, Q.; Gong, K.; Ao, Q.; Ma, T.; Yan, Y.F.; Gong, Y.D.; Zhang, X.F. Positive charge of chitosan retards blood coagulation on chitosan films. J. Biomater. Appl. 2013, 27, 1032–1045. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Li, Z.X.; Wang, S.; Liu, C.S.; Meng, X.H.; Liu, C.G.; Lv, Y.H.; Yu, L.J. Preparation and blood coagulation evaluation of chitosan microspheres. J. Mater. Sci.-Mater. Med. 2008, 19, 1371–1377. [Google Scholar] [CrossRef]
- Lin, J.H.; Chen, A.P.; Li, T.T.; Lin, M.C.; Lou, C.W. Antibacterial behavior and physical properties of silver nanoparticle-doped ecofriendly nonwoven fabrics. Cellulose 2014, 21, 1957–1964. [Google Scholar] [CrossRef]
- Chhatre, A.; Solasa, P.; Sakle, S.; Thaokar, R.; Mehra, A. Color and surface plasmon effects in nanoparticle systems: Case of silver nanoparticles prepared by microemulsion route. Colloid Surf. A-Physicochem. Eng. Asp. 2012, 404, 83–92. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.K.; Singh, R.P.; Pandey, A.C. Black pepper assisted biomimetic synthesis of silver nanoparticles. J. Alloy. Compd. 2010, 507, L13–L16. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Kneipp, K. Size-Dependent Shifts of Plasmon Resonance in Silver Nanoparticle Films Using Controlled Dissolution: Monitoring the Onset of Surface Screening Effects. J. Phys. Chem. C 2014, 118, 28075–28083. [Google Scholar] [CrossRef]
- Prashanth, K.V.H.; Kittur, F.S.; Tharanathan, R.N. Solid state structure of chitosan prepared under different N-deacetylating conditions. Carbohydr. Polym. 2002, 50, 27–33. [Google Scholar] [CrossRef]
- Islam, M.M.; Masum, S.M.; Rahman, M.M.; Molla, M.A.I.; Shaikh, A.A.; Roy, S.K. Preparation of Chitosan from Shrimp Shell and Investigation of Its Properties. Int. J. Basic Appl. Sci. 2011, 11, 70–80. [Google Scholar]
- Liu, C.H.; Yang, X.P.; Yuan, H.Y.; Zhou, Z.D.; Xiao, D. Preparation of silver nanoparticle and its application to the determination of ct-DNA. Sensors 2007, 7, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.B.; Lisa, S.M. Plasma components: Properties, differences, and uses. Transfusion 2012, 52, 9s–19s. [Google Scholar]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawena, L.; Sandhya, B.; Jenyuk, L.; Satoshi, T. Release of silver nanoparticles from fabrics during the course of sequential washing. Environ. Sci. Pollut. Res. Int. 2016, 23, 22810–22818. [Google Scholar]
- Ba, W. The ultrastructure of platelet pseudopodia and the adhesion of homologous platelets to tumour cells. Br. J. Exp. Pathol. 1970, 51, 570–580. [Google Scholar]
- Liao, C.C.; Hsieh, P.C.; Lin, T.K.; Lin, C.L.; Lo, Y.L.; Lee, S.C. Surgical treatment of spontaneous spinal epidural hematoma: A 5-year experience Clinical article. J. Neurosurg.-Spine 2009, 11, 480–486. [Google Scholar] [CrossRef]
- Wu, J.M.; Miao, M.S.; Guan, J.; Zhang, X.Z.; Li, R.X.; Ye, P. Experiment on Hemostasis for Wounds with Collagen Sponge. BME Clin. Med. 2002, 6, 11–13. [Google Scholar]
















| Nonwoven Composite | Polyacrylate Fiber (wt %) | Tencel® (wt %) | Area Weight (g/m2) | Punching Density (Punches/cm2) | Thickness (mm) |
|---|---|---|---|---|---|
| 10 PT | 10 | 90 | 100 | 100 | 0.8 |
| 20 PT | 20 | 80 | 100 | 100 | 0.8 |
| 30 PT | 30 | 70 | 100 | 100 | 0.8 |
| 40 PT | 40 | 60 | 100 | 100 | 0.8 |
| 50 PT | 50 | 50 | 100 | 100 | 0.8 |
| Escherichia coli (mm) | Staphylococcus aureus (mm) | |
|---|---|---|
| PT nonwoven composite | 0 ± 0 | 0 ± 0 |
| 2 h PT composite dressing | 0.9 ± 0.06 | 0.2 ± 0.01 |
| 4 h PT composite dressing | 1.2 ± 0.10 | 0.4 ± 0.04 |
| 6 h PT composite dressing | 1.9 ± 0.09 | 0.8 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-T.; Chen, A.-P.; Lai, M.-F.; Lin, M.-C.; Shiu, B.-C.; Lou, C.-W.; Lin, J.-H. Hemostasis Evaluation of Antibacterial and Highly Absorbent Composite Wound Dressings in Animal Hemostasis Models. Polymers 2022, 14, 1764. https://doi.org/10.3390/polym14091764
Shih Y-T, Chen A-P, Lai M-F, Lin M-C, Shiu B-C, Lou C-W, Lin J-H. Hemostasis Evaluation of Antibacterial and Highly Absorbent Composite Wound Dressings in Animal Hemostasis Models. Polymers. 2022; 14(9):1764. https://doi.org/10.3390/polym14091764
Chicago/Turabian StyleShih, Yu-Tung, An-Pang Chen, Mei-Feng Lai, Mei-Chen Lin, Bing-Chiuan Shiu, Ching-Wen Lou, and Jia-Horng Lin. 2022. "Hemostasis Evaluation of Antibacterial and Highly Absorbent Composite Wound Dressings in Animal Hemostasis Models" Polymers 14, no. 9: 1764. https://doi.org/10.3390/polym14091764
APA StyleShih, Y. -T., Chen, A. -P., Lai, M. -F., Lin, M. -C., Shiu, B. -C., Lou, C. -W., & Lin, J. -H. (2022). Hemostasis Evaluation of Antibacterial and Highly Absorbent Composite Wound Dressings in Animal Hemostasis Models. Polymers, 14(9), 1764. https://doi.org/10.3390/polym14091764