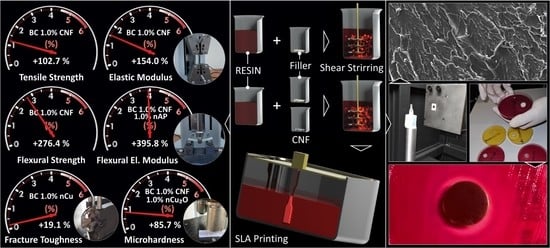
Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Nanocomposite Resin Mixtures and Fabrication of the 3D-Printed Specimens
2.3. Raman Spectroscopy and Thermal Analysis
2.4. Microstructural Investigations
2.5. Mechanical Testing
2.6. Biocidal Performance Investigation
3. Results and Discussion
3.1. Raman Spectroscopy and Thermal Analysis Results
3.2. Microstructural Investigations
3.3. Mechanical Testing Results
3.4. Biocidal Performance Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wimpenny, D.I.; Pandey, P.M.; Jyothish Kumar, L. Advances in 3D Printing & Additive Manufacturing Technologies; Springer: Singapore, 2016. [Google Scholar]
- Jyothish Kumar, L.; Pandey, P.M.; Wimpenny, D.I. 3D Printing and Additive Manufacturing Technologies; Springer: Singapore, 2018. [Google Scholar]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Yang, X.; Jones, A.; Zhang, J.; Park, H.Y.; Jung, Y.G. Additive manufacturing of polymer derived ceramics. Adv. Powder Metall. Part. Mater. 2020, 351, 716–725. [Google Scholar]
- Giannopoulos, A.A.; Mitsouras, D.; Yoo, S.J.; Liu, P.P.; Chatzizisis, Y.S.; Rybicki, F.J. Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 2016, 13, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Shahzad, A.; Lazoglu, I. Direct ink writing (DIW) of structural and functional ceramics: Recent achievements and future challenges. Compos. Part B Eng. 2021, 225, 109249. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Vairis, A.; Savvakis, K.; Maniadi, A. A parametric determination of bending and Charpy’s impact strength of ABS and ABS-plus fused deposition modeling specimens. Prog. Addit. Manuf. 2019, 4, 323–330. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of Stereolithography to Manufacture Critical-Sized 3D Biodegradable Scaffolds for Bone Ingrowth. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 64, 65–69. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Bao, H.; Liu, J.; Wu, Y.; Lai, G. Preparation and performance of ultraviolet curable silicone resins used for ultraviolet cured coating and ultraviolet-assisted 3D printing materials. OSA Contin. 2018, 1, 542. [Google Scholar] [CrossRef]
- Savvakis, K.; Petousis, M.; Vairis, A.; Vidakis, N.; Bikmeyev, A.T. Experimental determination of the tensile strength of fused deposition modeling parts. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Montreal, QC, Canada, 14–20 November 2014. [Google Scholar]
- De Schutter, G.; Lesage, K.; Mechtcherine, V.; Nerella, V.N.; Habert, G.; Agusti-Juan, I. Vision of 3D printing with concrete—Technical, economic and environmental potentials. Cem. Concr. Res. 2018, 112, 25–36. [Google Scholar] [CrossRef]
- Buchanan, C.; Gardner, L. Metal 3D printing in construction: A review of methods, research, applications, opportunities and challenges. Eng. Struct. 2019, 180, 332–348. [Google Scholar] [CrossRef]
- Walker, M.; Humphries, S. 3D Printing: Applications in evolution and ecology. Ecol. Evol. 2019, 9, 4289–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haselhuhn, A.S.; Wijnen, B.; Anzalone, G.C.; Sanders, P.G.; Pearce, J.M. In situ formation of substrate release mechanisms for gas metal arc weld metal 3-D printing. J. Mater. Process. Technol. 2015, 226, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Vairis, A.; Petousis, M.; Vidakis, N.; Savvakis, K. On the Strain Rate Sensitivity of Abs and Abs Plus Fused Deposition Modeling Parts. J. Mater. Eng. Perform. 2016, 25, 3558–3565. [Google Scholar] [CrossRef]
- Attaran, M. Additive Manufacturing: The Most Promising Technology to Alter the Supply Chain and Logistics. J. Serv. Sci. Manag. 2017, 10, 189–206. [Google Scholar] [CrossRef] [Green Version]
- Meyers, P.W.; Sivakumar, K.; Nakata, C. Implementation of Industrial Process Innovations: Factors, Effects, and Marketing Implications. J. Prod. Innov. Manag. 2003, 16, 295–311. [Google Scholar] [CrossRef]
- Rahman, A.A.; Bennett, D. Advanced manufacturing technology adoption in developing countries. The role of buyer-supplier relationships. J. Manuf. Technol. Manag. 2009, 20, 1099–1118. [Google Scholar] [CrossRef]
- Walker, J.L.; Santoro, M. Processing and Production of Bioresorbable Polymer Scaffolds for Tissue Engineering; Woodhead Publishing: Sawton, UK, 2017. [Google Scholar]
- Salonitis, K. Stereolithography. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Petousis, M.; Vidakis, N.; Velidakis, E.; Kechagias, J.D.; David, C.N.; Papadakis, S.; Mountakis, N. Affordable Biocidal Ultraviolet Cured Cuprous Oxide Filled Vat Photopolymerization Resin Nanocomposites with Enhanced Mechanical Properties. Biomimetics 2022, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Tsikritzis, D.; Gkagkanatsiou, A.; Kanellopoulou, S. Investigation of the Biocidal Performance of Multi-Functional Resin/Copper Nanocomposites with Superior Mechanical Response in SLA 3D Printing. Biomimetics 2022, 7, 8. [Google Scholar] [CrossRef]
- Holck, D.E.E.; Boyd, E.M.; Ng, J.; Mauffray, R.O. Benefits of stereolithography in orbital reconstruction. Ophthalmology 1999, 106, 1214–1218. [Google Scholar] [CrossRef]
- Peele, B.N.; Wallin, T.J.; Zhao, H.; Shepherd, R.F. 3D printing antagonistic systems of artificial muscle using projection stereolithography. Bioinspir. Biomimet. 2015, 10, 055003. [Google Scholar] [CrossRef]
- Ling, C.; Cernicchi, A.; Gilchrist, M.D.; Cardiff, P. Mechanical behaviour of additively-manufactured polymeric octet-truss lattice structures under quasi-static and dynamic compressive loading. Mater. Des. 2019, 162, 106–118. [Google Scholar] [CrossRef]
- Zhang, P.; To, A.C. Transversely isotropic hyperelastic-viscoplastic model for glassy polymers with application to additive manufactured photopolymers. Int. J. Plast. 2016, 80, 56–74. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, W. Photopolymerization and thermal behaviors of acrylated benzenephosphonates/epoxy acrylate as flame retardant resins. Eur. Polym. J. 2006, 42, 2261–2269. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Kechagias, J.D. A comprehensive investigation of the 3D printing parameters’ effects on the mechanical response of polycarbonate in fused filament fabrication. Prog. Addit. Manuf. 2022. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Liebscher, M.; Mechtcherine, V.; Tzounis, L. On the strain rate sensitivity of fused filament fabrication (Fff) processed pla, abs, petg, pa6, and pp thermoplastic polymers. Polymers 2020, 12, 2924. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Savvakis, K.; Maniadi, A.; Koudoumas, E. A comprehensive investigation of the mechanical behavior and the dielectrics of pure polylactic acid (PLA) and PLA with graphene (GnP) in fused deposition modeling (FDM). Int. J. Plast. Technol. 2019, 23, 195–206. [Google Scholar] [CrossRef]
- Vidakis, N.; Maniadi, A.; Petousis, M.; Vamvakaki, M.; Kenanakis, G.; Koudoumas, E. Mechanical and Electrical Properties Investigation of 3D-Printed Acrylonitrile–Butadiene–Styrene Graphene and Carbon Nanocomposites. J. Mater. Eng. Perform. 2020, 29, 1909–1918. [Google Scholar] [CrossRef]
- Tzounis, L.; Petousis, M.; Grammatikos, S.; Vidakis, N. 3D Printed Thermoelectric Polyurethane/Multiwalled Carbon Nanotube Nanocomposites: A Novel Approach towards the Fabrication of Flexible and Stretchable Organic Thermoelectrics. Materials 2020, 13, 2879. [Google Scholar] [CrossRef]
- Palaganas, J.O.; Palaganas, N.B.; Ramos, L.J.I.; David, C.P.C. 3D Printing of Covalent Functionalized Graphene Oxide Nanocomposite via Stereolithography. ACS Appl. Mater. Interfaces 2019, 11, 46034–46043. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, S.; Dhamodharan, D.; Divakaran, N.; Kale, M.B.; Senthil, T.; Wu, L.; Wang, J. Enhanced mechanical and thermal properties of stereolithography 3d printed structures by the effects of incorporated controllably annealed anatase TiO2 nanoparticles. Nanomaterials 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mubarak, S.; Dhamodharan, D.; Kale, M.B.; Divakaran, N.; Senthil, T.; Sathiyanathan, P.; Wu, L.; Wang, J. A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidakis, N.; Petousis, M.; Maniadi, A.; Koudoumas, E.; Liebscher, M.; Tzouins, L. Mechanical Properties of 3D-Printed Acrylonitrile–Butadiene–Styrene TiO2 and ATO Nanocomposites. Polymers 2021, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Liebscher, M.; Tzounis, L. Three-Dimensional Printed Antimicrobial Objects of Polylactic Acid (PLA)-Silver Nanoparticle Nanocomposite Filaments Produced by an In-Situ Reduction Reactive Melt Mixing Process. Biomimetics 2020, 5, 42. [Google Scholar] [CrossRef]
- Tzounis, L.; Bangeas, P.I.; Exadaktylos, A.; Petousis, M.; Vidakis, N. Three-dimensional printed polylactic acid (PLA) surgical retractors with sonochemically immobilized silver nanoparticles: The next generation of low-cost antimicrobial surgery equipment. Nanomaterials 2020, 10, 985. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Tzounis, L.; Liebscher, M.; Grammatikos, S.A. Enhanced mechanical, thermal and antimicrobial properties of additively manufactured polylactic acid with optimized nano silica content. Nanomaterials 2021, 11, 1012. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Chee, E.; Brown, A.C. Biomimetic antimicrobial material strategies for combating antibiotic resistant bacteria. Biomater. Sci. 2020, 8, 1089–1100. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, J.; Ning, X.; Cao, W.; Xu, W.; Guo, L. Controllable morphology and conductivity of electrodeposited Cu2O thin film: Effect of surfactants. ACS Appl. Mater. Interfaces 2014, 6, 22534–22543. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, P.; Sharma, A. Facile synthesis of Cu2O microstructures and their morphology dependent electrochemical supercapacitor properties. RSC Adv. 2016, 6, 3815–3822. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.C.; Sun, C.Y.; Chen, Z.X.; Wang, P.; Wang, H.T.; Yao, M.Z.; Wu, S.; Xu, P. Morphology control of Cu and Cu2O through electrodeposition on conducting polymer electrodes. Inorg. Chem. Front. 2021, 8, 1449–1454. [Google Scholar] [CrossRef]
- Krastev, E.T.; Voice, L.D.; Tobin, R.G. Surface morphology and electric conductivity of epitaxial Cu(100) films grown on H-terminated Si(100). J. Appl. Phys. 1996, 79, 6865–6871. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hou, X.; Lu, S.; Xu, W.; Tao, J.; Zhao, Z.; Hu, G.; Gao, F. Fabrication and simulation of a layered ultrahigh thermal conductive material made of self-assembled graphene and polydopamine on a copper substrate. RSC Adv. 2021, 11, 34676–34687. [Google Scholar] [CrossRef]
- Prakash, J.; Khan, S.; Chauhan, S.; Biradar, A.M. Metal oxide-nanoparticles and liquid crystal composites: A review of recent progress. J. Mol. Liq. 2020, 297, 112052. [Google Scholar] [CrossRef]
- Rashad, M. Tuning optical properties of polyvinyl alcohol doped with different metal oxide nanoparticles. Opt. Mater. 2020, 105, 109857. [Google Scholar] [CrossRef]
- Zhu, R.; Chung, C.H.; Cha, K.C.; Yang, W.; Zheng, Y.B.; Zhou, H.; Song, T.B.; Chen, C.C.; Weiss, P.S.; Li, G.; et al. Fused silver nanowires with metal oxide nanoparticles and organic polymers for highly transparent conductors. ACS Nano 2011, 5, 9877–9882. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef] [Green Version]
- Irvin, C.W.; Satam, C.C.; Carson Meredith, J.; Shofner, M.L. Mechanical reinforcement and thermal properties of PVA tricomponent nanocomposites with chitin nanofibers and cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2019, 116, 147–157. [Google Scholar] [CrossRef]
- Drogat, N.; Granet, R.; Sol, V.; Memmi, A.; Saad, N.; Klein Koerkamp, C.; Bressollier, P.; Krausz, P. Antimicrobial silver nanoparticles generated on cellulose nanocrystals. J. Nanoparticle Res. 2011, 13, 1557–1562. [Google Scholar] [CrossRef]
- Malmir, S.; Karbalaei, A.; Pourmadadi, M.; Hamedi, J.; Yazdian, F.; Navaee, M. Antibacterial properties of a bacterial cellulose CQD-TiO2 nanocomposite. Carbohydr. Polym. 2020, 234, 115835. [Google Scholar] [CrossRef]
- Pillai, K.V.; Renneckar, S. Dynamic mechanical analysis of layer-by-layer cellulose nanocomposites. Ind. Crops Prod. 2016, 93, 267–275. [Google Scholar] [CrossRef]
- Li, W.; Wu, Q.; Zhao, X.; Huang, Z.; Cao, J.; Li, J.; Liu, S. Enhanced thermal and mechanical properties of PVA composites formed with filamentous nanocellulose fibrils. Carbohydr. Polym. 2014, 113, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. A New Family of Nature-Based Materials. J. Ger. Chem. Soc. 2011, 50, 5438–5466. [Google Scholar]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Velidakis, E.; Mountakis, N.; Grammatikos, S.A. Polyamide 12/Multiwalled Carbon Nanotube and Carbon Black Nanocomposites Manufactured by 3D Printing Fused Filament Fabrication: A Comparison of the Electrical, Thermoelectric, and Mechanical Properties. C. 2021, 7, 38. [Google Scholar] [CrossRef]
- Graninger, G.; Kumar, S.; Garrett, G.; Falzon, B.G. Effect of shear forces on dispersion-related properties of microcrystalline cellulose-reinforced EVOH composites for advanced applications. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106103. [Google Scholar] [CrossRef]
- Bouzakis, K.D.; Vidakis, N. Superficial plastic response determination of hard isotropic materials using ball indentations and a FEM optimization technique. Mater. Charact. 1999, 42, 1–12. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymańska-Chargot, M.; Cybulska, J.; Zdunek, A. Sensing the structural differences in cellulose from apple and bacterial cell wall materials by Raman and FT-IR Spectroscopy. Sensors 2011, 11, 5543–5560. [Google Scholar] [CrossRef] [Green Version]
- Bichara, L.C.; Alvarez, P.E.; Fiori Bimbi, M.V.; Vaca, H.; Gervasi, C.; Brandán, S.A. Structural and spectroscopic study of a pectin isolated from citrus peel by using FTIR and FT-Raman spectra and DFT calculations. Infrared Phys. Technol. 2016, 76, 315–327. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence spectroscopy of polycarbonate matrices irradiated with different energy 28Si+ ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Zhang, S.; Bhagia, S.; Li, M.; Meng, X.; Ragauskas, A.J. Wood-reinforced composites by stereolithography with the stress whitening behavior. Mater. Des. 2021, 206, 109773. [Google Scholar] [CrossRef]
- Saba, N.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Mechanical, morphological and structural properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 97, 190–200. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, L.; Sun, J.; Liu, J. Cellulose Nanofibrils Filled Poly(Lactic Acid) Biocomposite Filament for FDM 3D Printing. Molecules 2020, 25, 2319. [Google Scholar] [CrossRef] [PubMed]
- Ambone, T.; Torris, A.; Shanmuganathan, K. Enhancing the mechanical properties of 3D printed polylactic acid using nanocellulose. Polym. Eng. Sci. 2020, 60, 1842–1855. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Mulholland, T.; Osswald, T.A. Characterization of mechanical properties and fracture mode of PLA and copper/PLA composite part manufactured by fused deposition modeling. SN Appl. Sci. 2019, 1, 616. [Google Scholar] [CrossRef] [Green Version]















| Wavenumber (cm−1) | Assignment |
|---|---|
| 450 | CCC, COC, OCC, OCO skeletal bending [70] |
| 531–534 | |
| 585 | Phenyl ring vibration [75] |
| 690 | Low-frequency vibrations of the pyranoid ring [71,72] |
| 1343 | C–C-H, C-O–H, and O-C-H [73] |
| 1523–1524 | –C=C- [74] |
| Material | Rq (nm) | Ra (nm) | Rz (nm) |
|---|---|---|---|
| BC 0 wt.% | 41.6 | 33.4 | 267.4 |
| BC 1 wt.% CNF | 176.5 | 145.7 | 1027.3 |
| BC 1 wt.% Cu | 64.7 | 50.7 | 450.8 |
| BC 1 wt.% Cu, 1 wt.% CNF | 39 | 28.5 | 386.6 |
| BC 1 wt.% Cu2O | 176.5 | 145.7 | 1027.3 |
| BC 1 wt.% CNF, 1 wt.% Cu2O | 45.6 | 34.9 | 451.9 |
| BC 1 wt.% AP | 267.5 | 218.9 | 1579.9 |
| BC 1 wt.% CNF, 1 wt.% AP | 145.3 | 115.3 | 1037.2 |
| Material | Tensile Toughness (MJ/m3) (Deviation) | Impact Toughness (MJ/m3) (Deviation) | Microhardness (HV) |
|---|---|---|---|
| BC 0 wt.% | 9.4 (1.0) | 2.7 (0.2) | 14.5 (0.8) |
| BC 1 wt.% CNF | 6.4 (0.4) | 1.9 (0.1) | 22.9 (0.8) |
| BC 1 wt.% Cu | 6.6 (0.8) | 3.1 (0.2) | 20.6 (1.0) |
| BC 1 wt.% Cu, 1 wt.% CNF | 4.1 (0.3) | 1.0 (0.1) | 24.8 (0.9) |
| BC 1 wt.% Cu2O | 3.4 (0.2) | 1.3 (0.2) | 19.9 (0.7) |
| BC 1 wt.% CNF, 1 wt.% Cu2O | 1.9 (0.2) | 0.6 (0.03) | 25.6 (0.8) |
| BC 1 wt.% AP | 4.6 (0.2) | 2.4 (0.03) | 21.8 (1.2) |
| BC 1 wt.% CNF, 1 wt.% AP | 6.3 (0.5) | 1.1 (0.2) | 22.3 (1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidakis, N.; Petousis, M.; Michailidis, N.; Papadakis, V.; Korlos, A.; Mountakis, N.; Argyros, A. Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents. Polymers 2022, 14, 1903. https://doi.org/10.3390/polym14091903
Vidakis N, Petousis M, Michailidis N, Papadakis V, Korlos A, Mountakis N, Argyros A. Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents. Polymers. 2022; 14(9):1903. https://doi.org/10.3390/polym14091903
Chicago/Turabian StyleVidakis, Nectarios, Markos Petousis, Nikolaos Michailidis, Vassilis Papadakis, Apostolos Korlos, Nikolaos Mountakis, and Apostolos Argyros. 2022. "Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents" Polymers 14, no. 9: 1903. https://doi.org/10.3390/polym14091903
APA StyleVidakis, N., Petousis, M., Michailidis, N., Papadakis, V., Korlos, A., Mountakis, N., & Argyros, A. (2022). Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents. Polymers, 14(9), 1903. https://doi.org/10.3390/polym14091903