Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
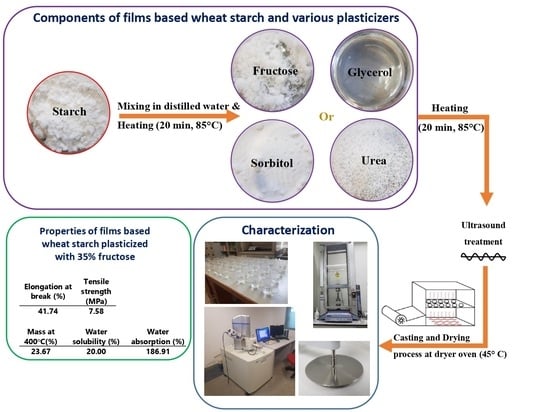
2.2. Film Preparation
2.3. Physical Properties
2.3.1. Film Thickness
2.3.2. Film Density
2.3.3. Moisture Content (MC)
2.3.4. Water Solubility (WS)
2.3.5. Water Absorption (WA)
2.4. Film Transparency
2.5. Biodegradation of Biocomposites (Soil Burial Test)
2.6. Simultaneous Thermal Analysis (STA)
2.7. Structural Properties
2.7.1. Scanning Electron Microscopy (SEM)
2.7.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.7.3. X-ray Diffraction (XRD)
2.8. Water Vapor Permeability (WVP)
2.9. Tensile Test
3. Results and Discussion
3.1. Physical Properties
3.1.1. Thickness and Density
3.1.2. Moisture Content and Water Solubility
3.1.3. Water Absorption
3.2. Film Transparency
3.3. Soil Degradation
3.4. Water Vapor Permeability
3.5. Structural Properties
3.5.1. Scanning Electron Microscopy (SEM)
3.5.2. Fourier Transform Infrared (FT-IR) Spectroscopy
3.5.3. X-ray Diffraction (XRD)
3.6. Simultaneous Thermal Analysis (STA)
3.7. Tensile Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- History and Future of Plastics|Science History Institute. Available online: https://www.sciencehistory.org/the-history-and-future-of-plastics (accessed on 10 August 2020).
- Plastic Pollution. Available online: https://www.unep.org/plastic-pollution (accessed on 20 October 2022).
- Hakeem, K.R.; Jawaid, M.; Rashid, U. Biomass and Bioenergy: Processing and Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–367. ISBN 9783319076423. [Google Scholar] [CrossRef]
- Jawaid, M.; Sapuan, S.M.; Alotman, O.Y. Green Biocomposites Manufacturing and Properties; Springer: Berlin/Heidelberg, Germany, 2017; p. 409. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.; Sain, M. Progress in Polymer Science Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Almajid, A.A. Manufacturing aspects of advanced polymer composites for automotive applications. Appl. Compos. Mater. 2013, 20, 107–128. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.; Abu Bakar, A.; Abdul Khalil, H.P.S.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef]
- Ho, M.P.; Wang, H.; Lee, J.H.; Ho, C.K.; Lau, K.T.; Leng, J.; Hui, D. Critical factors on manufacturing processes of natural fibre composites. Compos. Part B Eng. 2012, 43, 3549–3562. [Google Scholar] [CrossRef]
- Viana, E.B.M.; Leite, N.O.; Ribeiro, J.S.; Almeida, M.F.; Souza, C.C.E.; Resende, J.V.; Santos, L.S.; Veloso, C.M. Development of starch-based bioplastics of green plantain banana (Musa paradisiaca L.) modified with heat-moisture treatment (HMT). Food Packag. Shelf Life 2022, 31, 100776. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Production of Wheat Worldwide 2021/2022|Statista. Available online: https://www.statista.com/statistics/267268/production-of-wheat-worldwide-since-1990/ (accessed on 20 October 2022).
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Omran, A.A.B.; Hasan, Z.; Ilyas, R.A.; Sapuan, S.M. Wheat Biocomposite Extraction, Structure, Properties and Characterization: A Review. Polymers 2021, 13, 3624. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Hoag, S.W. Plasticizer effects on physical-mechanical properties of solvent cast Soluplus® films. AAPS PharmSciTech 2013, 14, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Dynamic Mechanical Properties of Sugar Palm Starch-based Films. Int. J. Polym. Anal. Charact. 2015, 20, 627–636. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Mechanical and Physical Properties of Pea Starch Edible Films in the Presence of Glycerol. J. Food Process. Preserv. 2016, 40, 1339–1351. [Google Scholar] [CrossRef]
- Lim, W.S.; Ock, S.Y.; Park, G.D.; Lee, I.W.; Lee, M.H.; Park, H.J. Heat-sealing property of cassava starch film plasticized with glycerol and sorbitol. Food Packag. Shelf Life 2020, 26, 100556. [Google Scholar] [CrossRef]
- Srikaeo, K.; Thongta, R. Effects of sugarcane, palm sugar, coconut sugar and sorbitol on starch digestibility and physicochemical properties of wheat based foods. Int. Food Res. J. 2015, 22, 923–929. [Google Scholar]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of pectin/starch blends plasticized with glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Scamparini, A.R.P. Sucrose and inverted sugar as plasticizer. Effect on cassava starch-gelatin film mechanical properties, hydrophilicity and water activity. Food Chem. 2007, 103, 255–262. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Mali, S.; Grossmann, M.V.E.; Yamashita, F.; García, M.A. Effects of plasticizers on the properties of oat starch films. Mater. Sci. Eng. C 2009, 29, 532–538. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.R.; Jian, R.; Zheng, P.; Yu, J.; Ma, X. Preparation and properties of glycerol plasticized-starch (GPS)/cellulose nanoparticle (CN) composites. Carbohydr. Polym. 2010, 79, 301–305. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Kumar, V.V.; Elfaghi, A.M.; Ilyas, R.A.; Sapuan, S.M. Corn: Its Structure, Polymer, Fiber, Composite, Properties, and Applications. Polymers 2022, 14, 4396. [Google Scholar] [CrossRef] [PubMed]
- Avaro, M.R.A.; Pan, Z.; Yoshida, T.; Wada, Y. Two Alternative Methods to Predict Amylose Content of Rice Grain by Using Tristimulus CIE Lab Values and Developing a Specific Color Board of Starch-Iodine Complex Solution. Plant Prod. Sci. 2011, 14, 164–168. [Google Scholar] [CrossRef]
- Lu, Y.; Weng, L.; Cao, X. Morphological, thermal and mechanical properties of ramie crystallites—Reinforced plasticized starch biocomposites. Carbohydr. Polym. 2006, 63, 198–204. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ascione, L.; Mistretta, M.C.; Rapisarda, M.; Rizzarelli, P. Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers 2020, 12, 753. [Google Scholar] [CrossRef] [Green Version]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of glycerol on physical and mechanical properties of wheat starch edible films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- Amin, A.M.M.; Sauid, S.M.; Musa, M.; Ku Hamid, K.H. The effect of glycerol content on mechanical properties, surface morphology and water absorption of thermoplastic films from tacca leontopetaloides starch. J. Teknol. 2017, 79, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of starch composition and molecular weight on physicochemical properties of biodegradable films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obasi, H.C.; Igwe, I.O.; Madufor, I.C. Effect of Soil Burial on Tensile Properties of Polypropylene/Plasticized Cassava Starch Blends. Adv. Mater. Sci. Eng. 2013, 2013, 326538. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Aymard, C.; Guilbert, S. Relative humidity and temperature effects on mechanical and water vapor barrier properties of myofibrillar protein-based films. Polym. Gels Networks 1997, 5, 1–15. [Google Scholar] [CrossRef]
- Gaudin, S.; Lourdin, D.; Forssell, P.; Colona, P. Antiplasticisation and oxygen permeability of starch-sorbitol films. Carbohydr. Polym. 2000, 43, 33–37. [Google Scholar] [CrossRef]
- Caicedo, C.; Díaz-Cruz, C.A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films. Materials 2022, 15, 1274. [Google Scholar] [CrossRef]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch–chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Lee, C.M.; Kubicki, J.D.; Fan, B.; Zhong, L.; Jarvis, M.C.; Kim, S.H. Hydrogen-Bonding Network and OH Stretch Vibration of Cellulose: Comparison of Computational Modeling with Polarized IR and SFG Spectra. J. Phys. Chem. B 2015, 119, 15138–15149. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Dahryn Trivedi, A.B.; Khemraj Bairwa, H.S. Fourier Transform Infrared and Ultraviolet-Visible Spectroscopic Characterization of Biofield Treated Salicylic Acid and Sparfloxacin. Nat. Prod. Chem. Res. 2015, 3, 2329–6836. [Google Scholar] [CrossRef]
- Jaafar, J.; Siregar, J.P.; Oumer, A.N.; Hamdan, M.H.M.; Tezara, C.; Salit, M.S. Experimental investigation on performance of short pineapple leaf fiber reinforced tapioca biopolymer composites. BioResources 2019, 13, 6341–6355. [Google Scholar] [CrossRef]
- Corsetti, S.; Zehentbauer, F.M.; McGloin, D.; Kiefer, J. Characterization of gasoline/ethanol blends by infrared and excess infrared spectroscopy. Fuel 2015, 141, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Orphanou, C.M. The detection and discrimination of human body fluids using ATR FT-IR spectroscopy. Forensic Sci. Int. 2015, 252, e10–e16. [Google Scholar] [CrossRef]
- Yaacob, B.; Amin, M.C.I.M.; Hashim, K.; Bakar, B.A. Optimization of reaction conditions for carboxymethylated sago starch. Iran. Polym. J. Engl. Ed. 2011, 20, 195–204. [Google Scholar]
- Naznin, M.; Abedin, M.Z. Effect of Sugar, Urea, and Molasses and the Influence of Radiation on the Plasticization of Acacia catechu Extract Incorporated Starch/Poly-(Vinyl Alcohol) Based Film. ISRN Polym. Sci. 2013, 2013, 593862. [Google Scholar] [CrossRef] [Green Version]
- Rajisha, K.R.; Deepa, B.; Pothan, L.A.; Thomas, S. 9—Thermomechanical and spectroscopic characterization of natural fibre composites. In Woodhead Publishing Series in Composites Science and Engineering; Zafeiropoulos, N.E., Ed.; Woodhead Publishing: Sawston, UK, 2011; ISBN 978-1-84569-742-6. [Google Scholar]
- Loganathan, S.; Valapa, R.B.; Mishra, R.K.; Pugazhenthi, G.; Thomas, S. Chapter 4—Thermogravimetric Analysis for Characterization of Nanomaterials . In Micro and Nano Technologies; Thomas, S., Thomas, R., Zachariah, A.K., Mishra, R.K.B.T.-T., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. ISBN 978-0-323-46139-9. [Google Scholar]
- Suppakul, P.; Chalernsook, B.; Ratisuthawat, B.; Prapasitthi, S.; Munchukangwan, N. Empirical modeling of moisture sorption characteristics and mechanical and barrier properties of cassava flour film and their relation to plasticizing-antiplasticizing effects. LWT-Food Sci. Technol. 2013, 50, 290–297. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef]
- Versino, F.; López, O.V.; García, M.A. Sustainable use of cassava (Manihot esculenta) roots as raw material for biocomposites development. Ind. Crops Prod. 2015, 65, 79–89. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 31 August 2021).
- Tan, S.X.; Andriyana, A.; Ong, H.C.; Lim, S.; Pang, Y.L.; Ngoh, G.C. A Comprehensive Review on the Emerging Roles of Nanofillers and Plasticizers towards Sustainable Starch-Based Bioplastic Fabrication. Polymers 2022, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; That, N.T.T.; Nguyen, N.T.; Nguyen, H.T. Development of Starch-Based Bioplastic from Jackfruit Seed. Adv. Polym. Technol. 2022, 2022, 6547461. [Google Scholar] [CrossRef]
- Ng, J.S.; Kiew, P.L.; Lam, M.K.; Yeoh, W.M.; Ho, M.Y. Preliminary evaluation of the properties and biodegradability of glycerol- and sorbitol-plasticized potato-based bioplastics. Int. J. Environ. Sci. Technol. 2022, 19, 1545–1554. [Google Scholar] [CrossRef]











| Film | Thickness, µm | Density, g/cm3 | Water Content, % | Water Solubility, % | WVP, 10−10 g·mm·s−1 m−2 Pa−1 | Crystallinity Index, % | Drying Time, hours |
|---|---|---|---|---|---|---|---|
| C | 170.20 | 1.32 | 11.86 | 2.54 | 1.12 | 18.90 | 6.00 |
| 15% F | 156.40 | 1.59 | 8.70 | 14.92 | 1.22 | 17.80 | 7.00 |
| 25% F | 177.60 | 1.47 | 8.17 | 22.62 | 1.49 | 16.50 | 15.00 |
| 35% F | 200.20 | 1.39 | 9.15 | 29.02 | 1.53 | 16.90 | 22.00 |
| 15% G | 176.60 | 1.55 | 12.14 | 12.92 | 0.91 | 14.50 | 7.00 |
| 25% G | 205.20 | 1.37 | 13.67 | 19.27 | 1.13 | 15.50 | 24.00 |
| 35% G | 207.60 | 1.34 | 20.58 | 20.00 | 1.39 | 16.70 | 44.00 |
| 15% S | 155.00 | 1.55 | 10.38 | 15.19 | 1.24 | 13.10 | 7.00 |
| 25% S | 175.20 | 1.49 | 10.47 | 22.06 | 1.45 | 14.90 | 15.00 |
| 35% S | 189.60 | 1.48 | 9.90 | 28.00 | 1.58 | 14.10 | 22.00 |
| 15% U | 170.20 | 1.48 | 11.48 | 13.29 | 1.66 | 18.80 | 20.00 |
| 25% U | 190.40 | 1.45 | 16.98 | 17.81 | 1.77 | 15.50 | 16.00 |
| 35% U | 207.60 | 1.07 | 21.53 | 20.42 | 1.88 | 17.30 | 38.00 |
| Sample | Opacity (A600/mm) |
|---|---|
| C | 0.808 |
| 15%F | 1.032 |
| 25%F | 0.620 |
| 35%F | 0.624 |
| 15%G | 0.926 |
| 25%G | 0.723 |
| 35%G | 0.655 |
| 15%S | 0.954 |
| 25%S | 0.615 |
| 35%S | 0.585 |
| 15%U | 0.766 |
| 25%U | 0.774 |
| 35%U | 1.806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.A.B.A.; Hasan, Z.; Omran, A.A.B.; Elfaghi, A.M.; Khattak, M.A.; Ilyas, R.A.; Sapuan, S.M. Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers 2023, 15, 63. https://doi.org/10.3390/polym15010063
Mohammed AABA, Hasan Z, Omran AAB, Elfaghi AM, Khattak MA, Ilyas RA, Sapuan SM. Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers. 2023; 15(1):63. https://doi.org/10.3390/polym15010063
Chicago/Turabian StyleMohammed, Abdulrahman A. B. A., Zaimah Hasan, Abdoulhdi A. Borhana Omran, Abdulhafid M. Elfaghi, M.A. Khattak, R. A. Ilyas, and S. M. Sapuan. 2023. "Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films" Polymers 15, no. 1: 63. https://doi.org/10.3390/polym15010063
APA StyleMohammed, A. A. B. A., Hasan, Z., Omran, A. A. B., Elfaghi, A. M., Khattak, M. A., Ilyas, R. A., & Sapuan, S. M. (2023). Effect of Various Plasticizers in Different Concentrations on Physical, Thermal, Mechanical, and Structural Properties of Wheat Starch-Based Films. Polymers, 15(1), 63. https://doi.org/10.3390/polym15010063