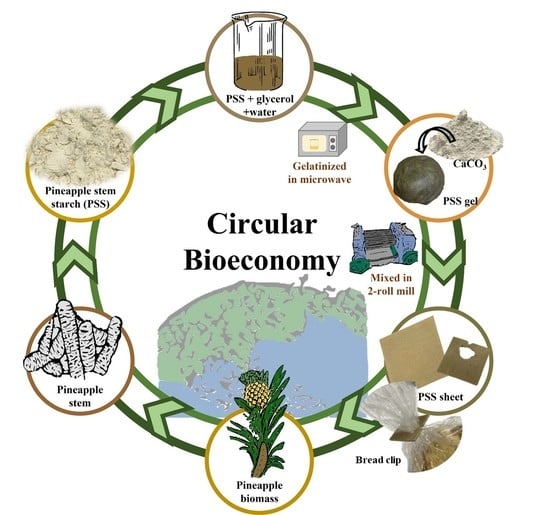
Toward a Circular Bioeconomy: Development of Pineapple Stem Starch Composite as a Plastic-Sheet Substitute for Single-Use Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Preparation of Starch Paste and Composites
2.3. Characterization of PSS Composites
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. X-ray Diffraction (XRD)
2.3.3. Mechanical Properties
2.3.4. Morphology
2.3.5. Water Solubility and Absorption
2.3.6. Soil Burial Test
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. X-ray Diffraction (XRD)
3.3. Mechanical Properties
3.4. Morphology
3.5. Water Solubility and Absorption
3.6. Soil Burial Test
4. Discussion and Potential Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K. The Countries Banning Plastic Bags. Available online: https://www.statista.com/chart/14120/the-countries-banning-plastic-bags/ (accessed on 25 April 2023).
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of poly(lactic acid) (PLA), Poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV blend in compost and soil environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Jia, M.Z. Biodegradable Plastics: Breaking Down the Facts, Greenpeace East Asia. 2020. Available online: https://www.greenpeace.org/static/planet4-eastasia-stateless/84075f56-biodegradable-plastics-report.pdf (accessed on 11 April 2023).
- Wang, B.; Yu, B.; Yuan, C.; Guo, L.; Liu, P.; Gao, W.; Li, D.; Cui, B.; Abd El-Aty, A.M. An overview on plasticized biodegradable corn starch-based films: The physicochemical properties and gelatinization process. Crit. Rev. Food. Sci. 2022, 62, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.J.F. Starch: Major sources, properties and applications as thermoplastic materials. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 321–342. [Google Scholar]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Biliaderis, C.G.; Ogawa, H.; Kawasaki, N. Biodegradable films made from low-density polyethylene (LDPE), rice starch and potato starch for food packaging applications: Part 1. Carbohydr. Polym. 1998, 36, 89–104. [Google Scholar] [CrossRef]
- Kaur, H.; Banipal, T.S.; Thakur, S.; Bakshi, M.S.; Kaur, G.; Singh, N. Novel Biodegradable Films with Extraordinary Tensile Strength and Flexibility Provided by Nanoparticles. ACS Sustain. Chem. Eng. 2012, 1, 127–136. [Google Scholar] [CrossRef]
- Leal, I.L.; da Silva Rosa, Y.C.; da Silva Penha, J.; Cruz Correia, P.R.; da Silva Melo, P.; Guimarães, D.H.; Barbosa, J.D.V.; Druzian, J.I.; Machado, B.A.S. Development and application starch films: PBAT with additives for evaluating the shelf life of Tommy Atkins mango in the fresh-cut state. J. Appl. Polym. Sci. 2019, 136, 48150. [Google Scholar] [CrossRef]
- La Fuente, C.I.A.; de Souza, A.T.; Tadini, C.C.; Augusto, P.E.D. Ozonation of cassava starch to produce biodegradable films. Int. J. Biol. Macromol. 2019, 141, 713–720. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-conventional starch sources. Curr. Opin. Food. Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Henning, F.G.; Ito, V.C.; Demiate, I.M.; Lacerda, L.G. Non-conventional starches for biodegradable films: A review focussing on characterisation and recent applications in food. Carbohydr. Polym. Tech. Appl. 2021, 4, 100157. [Google Scholar] [CrossRef]
- González-Soto, R.A.; Sánchez-Hernández, L.; Solorza-Feria, J.; Núñez-Santiago, C.; Flores-Huicochea, E.; Bello-Pérez, L.A. Resistant starch production from non-conventional starch sources by extrusion. Food. Sci. Tech. Int. 2006, 12, 5–11. [Google Scholar] [CrossRef]
- Souza, C.O.; Silva, L.T.; Silva, J.R.; López, J.A.; Veiga-Santos, P.; Druzian, J.I. Mango and acerola pulps as antioxidant additives in cassava starch bio-based film. J. Agr. Food. Chem. 2011, 59, 2248–2254. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Hasnain, A. Biodegradable film from mango kernel starch: Effect of plasticizers on physical, barrier, and mechanical properties. Starch Stärke 2016, 68, 919–928. [Google Scholar] [CrossRef]
- Alimi, B.A.; Workneh, T.S.; Zubair, B.A. Microstructural and physicochemical properties of biodegradable films developed from false banana (Ensete ventricosum) starch. Heliyon 2022, 8, e09148. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Lucas, A.A.; Tapia-Blácido, D.R. Bioactive films based on babassu mesocarp flour and starch. Food Hydrocoll. 2017, 70, 383–391. [Google Scholar] [CrossRef]
- Tanetrungroj, Y.; Prachayawarakorn, J. Effect of dual modification on properties of biodegradable crosslinked-oxidized starch and oxidized-crosslinked starch films. Int. J. Biol. Macromol. 2018, 120, 1240–1246. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Kansanthia, P. Characterization and properties of singly and dually modified hydrogen peroxide oxidized and glutaraldehyde crosslinked biodegradable starch films. Int. J. Biol. Macromol. 2022, 194, 331–337. [Google Scholar] [CrossRef] [PubMed]
- González-Soto, R.A.; Núñez-Santiago, M.C.; Bello-Pérez, L.A. Preparation and partial characterization of films made with dual-modified (acetylation and crosslinking) potato starch. J. Sci. Food Agr. 2019, 99, 3134–3141. [Google Scholar] [CrossRef]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef]
- Shanmathy, M.; Mohanta, M.; Thirugnanam, A. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite. Carbohydr. Polym. Technol. Appl. 2021, 2, 100173. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Alvarez, V.A. Cellulosic materials as natural fillers in starch-containing matrix-based films: A review. Polym. Bull. 2017, 74, 2401–2430. [Google Scholar] [CrossRef]
- Pongsuwan, C.; Boonsuk, P.; Sermwittayawong, D.; Aiemcharoen, P.; Mayakun, J.; Kaewtatip, K. Banana inflorescence waste fiber: An effective filler for starch-based bioplastics. Ind. Crop. Prod. 2022, 180, 114731. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, H.Y. Studies on stem bromelain and stem starch from pineapple plants. Taiwania 1972, 17, 266–276. [Google Scholar]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crop. Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Rinju, R.; Harikumaran-Thampi, B.-S. Characteristics of Starch Extracted from the Stem of Pineapple Plant (Ananas comosus)—An Agro Waste from Pineapple Farms. Braz. Arch. Biol. Tech. 2021, 64, e21190276. [Google Scholar] [CrossRef]
- Namphonsane, A.; Suwannachat, P.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M.; Amornsakchai, T. Toward a Circular Bioeconomy: Exploring Pineapple Stem Starch Film as a Plastic Substitute in Single Use Applications. Membranes 2023, 13, 458. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Mohd Nizam, N.H.; Mohammad Rawi, N.F.; Ramle, S.F.M.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Mohamad Kassim, M.H. Physical, thermal, mechanical, antimicrobial and physicochemical properties of starch based film containing aloe vera: A review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels. 2012, 5, 13. [Google Scholar] [CrossRef]
- Zhu, C.; Chiu, S.; Nakas, J.P.; Nomura, C.T. Bioplastics from waste glycerol derived from biodiesel industry. J. Appl. Polym. Sci. 2013, 130, 1–13. [Google Scholar] [CrossRef]
- Bilck, A.P.; Olivera Müller, C.M.; Olivato, J.B.; Mali, S.; Grossmann, M.V.E.; Yamashita, F. Using glycerol produced from biodiesel as a plasticiser in extruded biodegradable films. Polimeros 2015, 25, 331–335. [Google Scholar] [CrossRef]
- Mallick, P.K. 2.09—Particulate and Short Fiber Reinforced Polymer Composites. Compr. Compos. Mater. 2000, 2, 291–331. [Google Scholar]
- Rothon, R.; Paynter, C. Calcium carbonate fillers. In Fillers for Polymer Applications; Rothon, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 149–160. [Google Scholar]
- Barros, M.C.; Bello, P.M.; Bao, M.; Torrado, J.J. From waste to commodity: Transforming shells into high purity calcium carbonate. J. Clean. Prod. 2009, 17, 400–407. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Benelli, P.; Amante, E.R. A literature review on adding value to solid residues: Egg shells. J. Clean. Prod. 2013, 46, 42–47. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Falini, G.; Manara, S.; Fermani, S.; Roveri, N.; Goisis, M.; Manganelli, G.; Cassar, L. Polymeric admixtures effects on calcium carbonate crystallization: Relevance to cement industries and biomineralization. CrystEngComm 2007, 9, 1162–1170. [Google Scholar] [CrossRef]
- Myllärinen, P.; Buleon, A.; Lahtinen, R.; Forssell, P. The crystallinity of amylose and amylopectin films. Carbohydr. Polym. 2002, 48, 41–48. [Google Scholar] [CrossRef]
- Rindlav-Westling, A.; Stading, M.; Hermansson, A.M.; Gatenholm, P. Structure, mechanical and barrier properties of amylose and amylopectin films. Carbohydr. Polym. 1998, 36, 217–224. [Google Scholar] [CrossRef]
- Sarko, A.; Wu, H.-C.H. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch Stärke 1978, 30, 73–78. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ottenhof, M.-A.; Farhat, I.A. Starch retrogradation. Biotechnol. Genet. Eng. Rev. 2004, 21, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Okawa, Y.; Ninomiya, K.; Kumagai, H.; Kumagai, H. Evaluation and suppression of retrogradation of gelatinized rice starch. J. Nutr. Sci. Vitaminol. 2019, 65, S134–S138. [Google Scholar] [CrossRef] [PubMed]
- Fourati, Y.; Tarrés, Q.; Mutjé, P.; Boufi, S. PBAT/thermoplastic starch blends: Effect of compatibilizers on the rheological, mechanical and morphological properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Mazerolles, T.; Heuzey, M.-C.; Soliman, M.; Martens, H.; Kleppinger, R.; Huneault, M.A. Development of multilayer barrier films of thermoplastic starch and low-density polyethylene. J. Polym. Res. 2020, 27, 44. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- KLR Launches Sustainable Bag Clip. Available online: https://www.bakersjournal.com/klr-launches-sustainable-bag-clip-7854/ (accessed on 24 April 2023).












| No. | Observed Position (cm−1) | Functional Group |
|---|---|---|
| 1 | 3300 | O-H stretching |
| 2 | 2925 | C-H stretching |
| 3 | 1644 | C-O bending (associate with OH group) |
| 4 | 1453 | CH2 symmetric deformation |
| 5 | 1413 | CH2 symmetric scissoring |
| 6 | 1368 | C-H symmetric bending |
| 7 | 1150 | C-O-C asymmetric stretching |
| 8 | 1076, 993 | C-O stretching |
| 9 | 923, 860, 760 | C-O-C ring vibration of carbohydrate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongphang, C.; Namphonsane, A.; Thanawan, S.; Chia, C.H.; Wongsagonsup, R.; Smith, S.M.; Amornsakchai, T. Toward a Circular Bioeconomy: Development of Pineapple Stem Starch Composite as a Plastic-Sheet Substitute for Single-Use Applications. Polymers 2023, 15, 2388. https://doi.org/10.3390/polym15102388
Thongphang C, Namphonsane A, Thanawan S, Chia CH, Wongsagonsup R, Smith SM, Amornsakchai T. Toward a Circular Bioeconomy: Development of Pineapple Stem Starch Composite as a Plastic-Sheet Substitute for Single-Use Applications. Polymers. 2023; 15(10):2388. https://doi.org/10.3390/polym15102388
Chicago/Turabian StyleThongphang, Chanaporn, Atitiya Namphonsane, Sombat Thanawan, Chin Hua Chia, Rungtiwa Wongsagonsup, Siwaporn Meejoo Smith, and Taweechai Amornsakchai. 2023. "Toward a Circular Bioeconomy: Development of Pineapple Stem Starch Composite as a Plastic-Sheet Substitute for Single-Use Applications" Polymers 15, no. 10: 2388. https://doi.org/10.3390/polym15102388
APA StyleThongphang, C., Namphonsane, A., Thanawan, S., Chia, C. H., Wongsagonsup, R., Smith, S. M., & Amornsakchai, T. (2023). Toward a Circular Bioeconomy: Development of Pineapple Stem Starch Composite as a Plastic-Sheet Substitute for Single-Use Applications. Polymers, 15(10), 2388. https://doi.org/10.3390/polym15102388