Synergistic Improvement of Flame Retardancy and Mechanical Properties of Epoxy/Benzoxazine/Aluminum Trihydrate Adhesive Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
3. Results
3.1. Thermal, Mechanical, Flammable Properties of Epxoy/Benzoxazine Mixtures
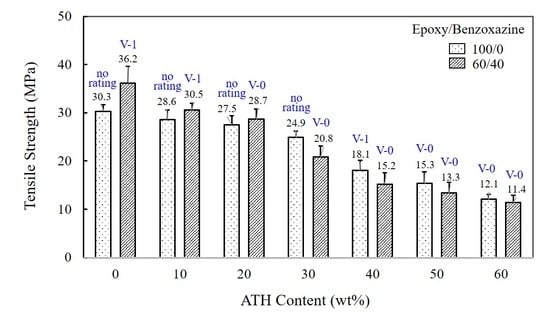
3.2. Thermal, Mechanical, and Flammable Properties of Epoxy/Benzoxazine/ATH Composites
3.3. Effect of Surface Modification on Mechanical Properties of Epoxy/Benzoxazine/ATH Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fulik, N.; Hofmann, A.; Nötzel, D.; Müller, M.; Reuter, I.; Müller, F.; Smith, A.; Hanemann, T. Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries. Batteries 2023, 9, 82. [Google Scholar] [CrossRef]
- Kovács, Z.; Polmázi, Á.; Toldy, A. Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications. Coatings 2023, 13, 345. [Google Scholar] [CrossRef]
- Xiong, X.; Niu, Y.; Zhou, Z.; Ren, J. Development and Application of a New Flame-Retardant Adhesive. Polymers 2020, 12, 2007. [Google Scholar] [CrossRef]
- Mariappan, T.; Wilkie, C.A. Flame retardant epoxy resin for electrical and electronic applications. Fire Mater. 2014, 38, 588–598. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, F.; Moon, K.S.; Wong, C.P. Novel Curing Agent for Lead-Free Electronics: Amino Acid. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1020–1027. [Google Scholar] [CrossRef]
- Machado, I.; Shaer, C.; Hurdle, K.; Calado, V.; Ishida, H. Towards the Development of Green Flame Retardancy by Polybenzoxazines. Prog. Polym. Sci. 2021, 121, 101435. [Google Scholar] [CrossRef]
- Zeng, M.; Zhu, W.; Feng, Z.; Chen, J.; Huang, Y.; Xu, Q.; Wang, J. Two novel halogen-free, phosphorus-free, and intrinsically flame-retardant benzoxazine thermosets containing electron-withdrawing bridge groups. J. Appl. Polym. Sci. 2020, 137, e49300. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhang, Y.; Ishida, H. Intrinsically noncombustible polymers without flame retardant additives: Sulfur-containing and bio-based benzoxazines. Eur. Polym. J. 2020, 133, 109770. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resins, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-H.; Hon, J.-M.; Wang, M.-W.; Juang, T.-Y. First halogen and phosphorus-free, flame-retardant benzoxazine thermosets derived from main-chain type bishydroxydeoxybenzoin-based benzoxazine polymers. Polymer 2018, 154, 35–41. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Korotkov, R.F.; Shutov, V.V.; Sirotin, I.S.; Gorbunova, I.Y. Benzoxazine Copolymers with Mono- and Difunctional Epoxy Active Diluents with Enhanced Tackiness and Reduced Viscosity. J. Compos. Sci. 2021, 5, 250. [Google Scholar] [CrossRef]
- Yue, J.; Wang, H.; Zhou, Q.; Zhao, P. Reaction-Induced Phase Separation and Morphology Evolution of Benzoxazine/Epoxy/Imidazole Ternary Blends. Polymers 2021, 13, 2945. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, J.; Zhou, J.; Zhang, D.; Zhang, A. Dramatic toughness enhancement of benzoxazine/epoxy thermosets with a novel hyperbranched polymeric ionic liquid. Chem. Eng. J. 2018, 334, 1371–1382. [Google Scholar] [CrossRef]
- Chow, W.S.; Grishchuk, S.; Burkhart, T.; Karger-Kocsis, J. Gelling and curing behaviors of benzoxazine/epoxy formulations containing 4,4′-thiodiphenol accelerator. Thermochim. Acta 2012, 543, 172–177. [Google Scholar]
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on Persistent Organic Pollutants(Recast); European Union: Brussels, Belgium, 2019. [Google Scholar]
- Yang, X.; Shen, A.; Su, Y.; Zhao, W. Effects of alumina trihydrate (ATH) and organic montmorillonite (OMMT) on asphalt fume emission and flame retardancy properties of SBS-modified asphalt. Constr. Build. Mater. 2020, 236, 117576. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Li, L. Flame Retardant Behavior of Ternary Synergistic Systems in Rigid Polyurethane Foams. Polymers 2019, 11, 207. [Google Scholar]
- Vaari, J.; Paajanen, A. Evaluation of the reactive molecular dynamics method for Research on flame retardants: ATH-filled polyethylene. Comput. Mater. Sci. 2018, 153, 103–112. [Google Scholar]
- Elbasuney, S. Novel multi-component flame retardant system based on nanoscopic aluminium-trihydroxide (ATH). Powder Technol. 2017, 305, 538–545. [Google Scholar]
- Han, Z.; Wang, Y.; Dong, W.; Wang, P. Enhanced fire retardancy of polyethylene/alumina trihydrate composites by graphene nanoplatelets. Mater. Lett. 2014, 128, 275–278. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Y.; Kuai, D. Preparation and flame retardant property of nano-aluminum hydroxide foam for preventing spontaneous coal combustion. Fuel 2021, 304, 121494. [Google Scholar] [CrossRef]
- Qin, Z.; Li, D.; Li, Q.; Yang, R. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Daimatsu, K.; Sugimoto, H.; Kato, Y.; Nakanishi, E.; Inomata, K.; Amekawa, Y.; Takemura, K. Preparation and physical properties of flame retardant acrylic resin containing nano-sized aluminum hydroxide. Polym. Degrad. Stab. 2007, 92, 1433–1438. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Taraghi, I.; Piesowicz, E.; Sieminski, J.; Zawisza, K.; Pypeć, K.; Dobrzynska, R.; Terelak-Tymczyna, A.; Stateczny, K.; et al. Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation. Polymers 2021, 13, 2134. [Google Scholar] [CrossRef]
- Wu, B.; Kong, W.; Hu, K.; Fu, X.; Lei, J.; Zhou, C. Synergistic effect of phosphorus-containing silane coupling agent with alumina trihydrate in ethylene-vinyl acetate composites. Adv. Polym. Technol. 2018, 37, 1456–1468. [Google Scholar] [CrossRef]
- Lin, H.; Yan, H.; Liu, B.; Wei, L.; Xu, B. The influence of KH-550 on properties of ammonium polyphosphate and polypropylene flame retardant composites. Polym. Degrad. Stab. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.Z.; Zhou, Q.; Shao, W. Aluminum hydroxide filled ethylene vinyl acetate (EVA) composites: Effect of the interfacial compatibilizer and the particle size. J. Mater. Sci. 2007, 42, 4227–4232. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef]
- Amirbeygi, H.; Khosravi, H.; Tohidlou, E. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar] [CrossRef]
- Choi, S.; Maul, S.; Stewart, A.; Hamilton, H.R.; Douglas, E.P. Effect of Silane Coupling Agent on the Durability of Epoxy Adhesion for Structural Strengthening Applications. Polym. Eng. Sci. 2013, 53, 283–294. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Greiner, L.; Schönberger, F.; Döring, M. Synergistic flame retardant interplay of phosphorus containing flame retardants with aluminum trihydrate depending on the specific surface area in unsaturated polyester resin. J. Appl. Polym. Sci. 2019, 136, 47270. [Google Scholar] [CrossRef]
- Yen, Y.-Y.; Wang, H.T.; Guo, W.J. Synergistic Effect of Aluminum Hydroxide and Nanoclay on Flame Retardancy and Mechanical Properties of EPDM Composites. J. Appl. Polym. Sci. 2013, 130, 2042–2048. [Google Scholar] [CrossRef]
- Schartel, B.; Knoll, U.; Hartwig, A.; Pütz, D. Phosphonium-modified layered silicate epoxy resins nanocomposites and their combinations with ATH and organo-phosphorus fire retardants. Polym. Adv. Technol. 2006, 17, 281–293. [Google Scholar] [CrossRef]







| Epoxy/Benzoxazine (wt/wt%) | Td (°C) | Tg (°C) | CTE (ppm, °C−1) | Modulus (GPa) | Viscosity (mPa∙s, 5 rpm) | ||
|---|---|---|---|---|---|---|---|
| α1 | α2 | 25 °C | 250 °C | ||||
| 100/0 | 301 | 159 | 95 | 162 | 5.2 | 0.015 | 250 |
| 90/10 | 300 | 159 | 70 | 161 | 5.2 | 0.019 | 663 |
| 80/20 | 355 | 161 | 68 | 153 | 5.3 | 0.015 | 2568 |
| 70/30 | 355 | 163 | 45 | 150 | 6.2 | 0.025 | 3397 |
| 60/40 | 350 | 161 | 37 | 141 | 5.2 | 0.030 | 17,170 |
| 50/50 | 354 | 163 | 35 | 142 | 4.8 | 0.031 | 29,830 |
| Epoxy/Benzoxazine (wt/wt%) | Combustion Time (s) | Dripping | Rating | |
|---|---|---|---|---|
| After 1st Ignition | After 2nd Ignition | |||
| 100/0 | burned | - | D | No rating |
| 90/10 | burned | - | D | No rating |
| 80/20 | burned | - | D | No rating |
| 70/30 | burned | - | D | No rating |
| 60/40 | 175 | 0 | N | V-1 |
| 50/50 | 71 | 0 | N | V-1 |
| ATH Content (wt%) | Tg (°C) | CTE (ppm, °C−1) | Modulus (GPa) | Viscosity (mPa∙s, 2 rpm) | ||
|---|---|---|---|---|---|---|
| α1 | α2 | 25 °C | 250 °C | |||
| 0 | 160.4 | 37 | 141 | 5.2 | 0.030 | 2278 |
| 10 | 162.4 | 33 | 133 | 7.2 | 0.045 | 3107 |
| 20 | 160.0 | 32 | 130 | 8.4 | 0.063 | 4142 |
| 30 | 159.2 | 32 | 109 | 8.4 | 0.065 | 6006 |
| 40 | 161.6 | 31 | 92 | 8.1 | 0.081 | 11,180 |
| Epoxy/Benzoxazine (wt/wt%) | ATH Content (wt%) | Combustion Time (s) | Dripping | Rating | |
|---|---|---|---|---|---|
| After 1st Ignition | After 2nd Ignition | ||||
| 100/0 | 10 | burned | - | D | No rating |
| 20 | burned | - | D | No rating | |
| 30 | burned | - | D | No rating | |
| 40 | 0 | 53 | N | V-1 | |
| 50 | 0 | 0 | N | V-0 | |
| 60 | 0 | 0 | N | V-0 | |
| 60/40 | 10 | 0 | 70 | N | V-1 |
| 20 | 0 | 35 | N | V-0 | |
| 30 | 0 | 25 | N | V-0 | |
| 40 | 0 | 0 | N | V-0 | |
| 50 | 0 | 0 | N | V-0 | |
| 60 | 0 | 0 | N | V-0 | |
| 50/50 | 10 | 0 | 40 | N | V-0 |
| 20 | 0 | 20 | N | V-0 | |
| 30 | 0 | 0 | N | V-0 | |
| 40 | 0 | 0 | N | V-0 | |
| 50 | 0 | 0 | N | V-0 | |
| 60 | 0 | 0 | N | V-0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, K.-S.; Kim, N. Synergistic Improvement of Flame Retardancy and Mechanical Properties of Epoxy/Benzoxazine/Aluminum Trihydrate Adhesive Composites. Polymers 2023, 15, 2452. https://doi.org/10.3390/polym15112452
Sung K-S, Kim N. Synergistic Improvement of Flame Retardancy and Mechanical Properties of Epoxy/Benzoxazine/Aluminum Trihydrate Adhesive Composites. Polymers. 2023; 15(11):2452. https://doi.org/10.3390/polym15112452
Chicago/Turabian StyleSung, Kyung-Soo, and Namil Kim. 2023. "Synergistic Improvement of Flame Retardancy and Mechanical Properties of Epoxy/Benzoxazine/Aluminum Trihydrate Adhesive Composites" Polymers 15, no. 11: 2452. https://doi.org/10.3390/polym15112452
APA StyleSung, K. -S., & Kim, N. (2023). Synergistic Improvement of Flame Retardancy and Mechanical Properties of Epoxy/Benzoxazine/Aluminum Trihydrate Adhesive Composites. Polymers, 15(11), 2452. https://doi.org/10.3390/polym15112452