Super-Tough and Biodegradable Poly(lactide-co-glycolide) (PLGA) Transparent Thin Films Toughened by Star-Shaped PCL-b-PDLA Plasticizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
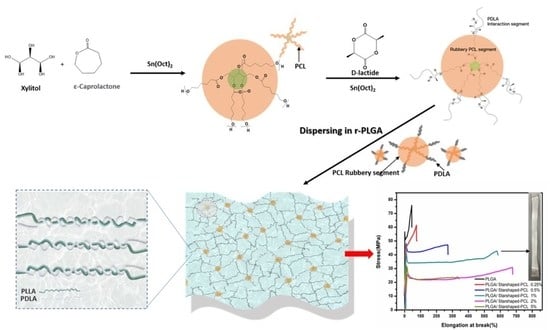
2.2. Experimental
2.2.1. Synthesis of Random Poly(lactide-co-glycolide) Copolymer (PLGA)
2.2.2. Synthesis of Star-Shaped PCL-b-PDLA Plasticizers
2.2.3. Preparation of PLGA/Star-Shaped PCL-b-PDLA Thin Films
2.2.4. Characterization
3. Results and Discussion
3.1. Polymerization and Characterization of PLGA
3.2. Preparation and Characterization of Star-Shaped Poly(ε-caprolactone-co-D-lactide)
3.2.1. Mechanical Properties
3.2.2. Thermal Properties
3.2.3. Morphology
3.2.4. Transparency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef] [Green Version]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, Y.D.; Zeng, J.B. Progress in Toughening Poly(Lactic Acid) with Renewable Polymers. Polym. Rev. 2017, 57, 557–593. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Hu, J.; Wu, Z.; Chen, H. Fabrication and Characterization of Polylactic Acid/Polycaprolactone Composite Macroporous Micro-Nanofiber Scaffolds by Phase Separation. New J. Chem. 2020, 44, 17382–17390. [Google Scholar] [CrossRef]
- Silva, A.T.C.R.; Cardoso, B.C.O.; e Silva, M.E.S.R.; Freitas, R.F.S.; Sousa, R.G. Synthesis, Characterization, and Study of PLGA Copolymer in Vitro Degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Mulchandani, N.; Masutani, K.; Kumar, S.; Yamane, H.; Sakurai, S.; Kimura, Y.; Katiyar, V. Toughened PLA-b-PCL-b-PLA Triblock Copolymer Based Biomaterials: Effect of Self-Assembled Nanostructure and Stereocomplexation on the Mechanical Properties. Polym. Chem. 2021, 12, 3806–3824. [Google Scholar] [CrossRef]
- Gao, J.; Chen, S.; Tang, D.; Jiang, L.; Shi, J.; Wang, S. Mechanical Properties and Degradability of Electrospun PCL/PLGA Blended Scaffolds as Vascular Grafts. Trans. Tianjin Univ. 2019, 25, 152–160. [Google Scholar] [CrossRef]
- Little, A.; Wemyss, A.M.; Haddleton, D.M.; Tan, B.; Sun, Z.; Ji, Y.; Wan, C. Synthesis of Poly(Lactic Acid-Co-Glycolic Acid) Copolymers with High Glycolide Ratio by Ring-Opening Polymerisation. Polymers 2021, 13, 2458. [Google Scholar] [CrossRef]
- Ayyoob, M.; Kim, Y.J. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on Thewettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers 2018, 10, 1132. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Pang, D.; Ma, C.; Li, Q.; Xiong, C. Composites of Hydroxyapatite Whiskers/Poly(L-Lactide-Co-Glycolide) with High Tensile Plasticity. J. Macromol. Sci. Part B Phys. 2012, 51, 1242–1255. [Google Scholar] [CrossRef]
- Park, J.; Kang, S. A Study on Surface, Thermal and Mechanical Properties of Absorbable PLGA Plate. Int. J. Control Autom. 2013, 6, 73–82. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, H.; Xu, Y.; Yang, J.; Zhou, X.; Zhang, F.; Gu, N. The Preosteoblast Response of Electrospinning PLGA/PCL Nanofibers: Effects of Biomimetic Architecture and Collagen I. Int. J. Nanomed. 2016, 11, 4157–4171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA Composite Membranes for Potential Application in Guided Tissue Regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Yu, E.J.; Lee, W.K.; Ha, C.S. Mechanical Properties and Degradation Studies of Poly(D,L-Lactide-Co-Glycolide) 50:50/Graphene Oxide Nanocomposite Films. Polym. Adv. Technol. 2014, 25, 48–54. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Chen, H.; Zhang, S.; Xiong, C. Preparation and Characterization of Biodegradable Thermoplastic Elastomers (PLCA/PLGA Blends). J. Polym. Res. 2010, 17, 77–82. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Garcia-Garcia, P.; Gutierrez, F.B.; Aguirre, J.J.; Hernandez, R.M.; Delgado, A.; Igartua, M. Composite Nanofibrous Membranes of PLGA/Aloe Vera Containing Lipid Nanoparticles for Wound Dressing Applications. Int. J. Pharm. 2019, 556, 320–329. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA Membrane Incorporated with Andrographolide-Loaded Mesoporous Silica Nanoparticles for Sustained Antibacterial Wound Dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; ur Rehman, A. Recent Applications of PLGA Based Nanostructures in Drug Delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Yen, T.T.H.; Linh, D.T.; Minh Hue, P.T. The Application of Microfluidics in Preparing Nano Drug Delivery Systems. VNU J. Sci. Med. Pharm. Sci. 2019, 35, 547–562. [Google Scholar] [CrossRef]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of Electrospun PCL/Gelatin Nanofibrous Scaffold for Wound Healing and Layered Dermal Reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun Nanofibrous Polyurethane Membrane as Wound Dressing. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Ghubayra, R.; Carpenter-Thompson, R.D.; Jiao, T.; Li, B. Solvent Selection and Its Effect on Crystallization Behavior of Poly(ε-Caprolactone) in Electrospun Poly(ε-Caprolactone)/Poly (Lactic-Co-Glycolic Acid) Blend Fibers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128896. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, T.G. Synthesis and Characterization of Elastic PLGA/PCL/PLGA Tri-Block Copolymers. J. Biomater. Sci. Polym. Ed. 2002, 13, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Feng, S.; Liu, X.; Xing, L.; Chen, D.; Xiong, C. Morphology, Thermal Properties, Mechanical Property and Degradation of PLGA/PTMC Composites. J. Polym. Res. 2020, 27, 387. [Google Scholar] [CrossRef]
- Naik, A.; Best, S.M.; Cameron, R.E. The Influence of Silanisation on the Mechanical and Degradation Behaviour of PLGA/HA Composites. Mater. Sci. Eng. C 2015, 48, 642–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuyun, J.; Chengdong, X.; Dongliang, C.; Lixin, J.; Xiubing, P. Effect of N-HA with Different Surface-Modified on the Properties of n-HA/PLGA Composite. Appl. Surf. Sci. 2012, 259, 72–78. [Google Scholar] [CrossRef]
- Duan, B.; Yuan, X.; Zhu, Y.; Zhang, Y.; Li, X.; Zhang, Y.; Yao, K. A Nanofibrous Composite Membrane of PLGA-Chitosan/PVA Prepared by Electrospinning. Eur. Polym. J. 2006, 42, 2013–2022. [Google Scholar] [CrossRef]
- Hiep, N.T.; Lee, B.T. Electro-Spinning of PLGA/PCL Blends for Tissue Engineering and Their Biocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 1969–1978. [Google Scholar] [CrossRef]
- Torres, F.G.; Nazhat, S.N.; Sheikh Md Fadzullah, S.H.; Maquet, V.; Boccaccini, A.R. Mechanical Properties and Bioactivity of Porous PLGA/TiO2 Nanoparticle-Filled Composites for Tissue Engineering Scaffolds. Compos. Sci. Technol. 2007, 67, 1139–1147. [Google Scholar] [CrossRef]
- Sun, Y.; He, C. Synthesis and Stereocomplex Crystallization of Poly(Lactide)-Graphene Oxide Nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Bai, T.; Wang, P.; Wang, Y.; Liu, C.; Shen, C. Selective Dispersion of Carbon Nanotubes and Nanoclay in Biodegradable Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends with Improved Toughness, Strength and Thermal Stability. Int. J. Biol. Macromol. 2020, 153, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Doganci, M.D.; Caner, D.; Doganci, E.; Ozkoc, G. Effects of Hetero-Armed Star-Shaped PCL-PLA Polymers with POSS Core on Thermal, Mechanical, and Morphological Properties of PLA. J. Appl. Polym. Sci. 2021, 138, 50712. [Google Scholar] [CrossRef]
- Li, A.D.; Sun, Z.Z.; Zhou, M.; Xu, X.X.; Ma, J.Y.; Zheng, W.; Zhou, H.M.; Li, L.; Zheng, Y.F. Electrospun Chitosan-Graft-PLGA Nanofibres with Significantly Enhanced Hydrophilicity and Improved Mechanical Property. Colloids Surf. B Biointerfaces 2013, 102, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly Biodegradable and Tough Polylactic Acid–Cellulose Nanocrystal Composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- González-Benito, J.; Castillo, E.; Caldito, J.F. Coefficient of Thermal Expansion of TiO2 Filled EVA Based Nanocomposites. A New Insight about the Influence of Filler Particle Size in Composites. Eur. Polym. J. 2013, 49, 1747–1752. [Google Scholar] [CrossRef]
- Forati, T.; Atai, M.; Rashidi, A.M.; Imani, M.; Behnamghader, A. Physical and Mechanical Properties of Graphene Oxide/Polyethersulfone Nanocomposites. Polym. Adv. Technol. 2014, 25, 322–328. [Google Scholar] [CrossRef]
- Li, Z.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C. Biodegradable Silica Rubber Core-Shell Nanoparticles and Their Stereocomplex for Efficient PLA Toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Yin, B.; Hakkarainen, M. Core-Shell Nanoparticle-Plasticizers for Design of High-Performance Polymeric Materials with Improved Stiffness and Toughness. J. Mater. Chem. 2011, 21, 8670–8677. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA-PHB/Cellulose Nanocrystal Films: Processing, Structural and Thermal Properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Ochi, M.; Takahashi, R.; Terauchi, A. Phase Structure and Mechanical and Adhesion Properties of Epoxy/Silica Hybrids. Polymer 2001, 42, 5151–5158. [Google Scholar] [CrossRef]
- Gardella, L.; Calabrese, M.; Monticelli, O. PLA Maleation: An Easy and Effective Method to Modify the Properties of PLA/PCL Immiscible Blends. Colloid Polym. Sci. 2014, 292, 2391–2398. [Google Scholar] [CrossRef]
- Kim, J.A.; Seong, D.G.; Kang, T.J.; Youn, J.R. Effects of Surface Modification on Rheological and Mechanical Properties of CNT/Epoxy Composites. Carbon 2006, 44, 1898–1905. [Google Scholar] [CrossRef]
- Patrício, T.; Bártolo, P. Thermal Stability of PCL/PLA Blends Produced by Physical Blending Process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Liang, J.Z.; Duan, D.R.; Tang, C.Y.; Tsui, C.P.; Chen, D.Z. Tensile Properties of PLLA/PCL Composites Filled with Nanometer Calcium Carbonate. Polym. Test. 2013, 32, 617–621. [Google Scholar] [CrossRef]
- Pereira Barros, J.J.; Dayane dos Santos Silva, I.; Jaques, N.G.; Lia Fook, M.V.; Ramos Wellen, R.M. Influence of PCL on the Epoxy Workability, Insights from Thermal and Spectroscopic Analyses. Polym. Test. 2020, 89, 106679. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Zhang, M.; Yu, W. Selective Localization of Multiwalled Carbon Nanotubes in Poly(ε-Caprolactone)/Polylactide Blend. Biomacromolecules 2009, 10, 417–424. [Google Scholar] [CrossRef]
- Matta, A.K.; Rao, R.U.; Suman, K.N.S.; Rambabu, V. Preparation and Characterization of Biodegradable PLA/PCL Polymeric Blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef] [Green Version]
- Forouharshad, M.; Gardella, L.; Furfaro, D.; Galimberti, M.; Monticelli, O. A Low-Environmental-Impact Approach for Novel Bio-Composites Based on PLLA/PCL Blends and High Surface Area Graphite. Eur. Polym. J. 2015, 70, 28–36. [Google Scholar] [CrossRef]
- Xie, M.-M.; Wang, B.-B.; Zhang, P. The Effect of Crystallization Behavior on High Conductivity, Enhanced Mechanism and Thermal Stability of Poly(ε-Caprolactone)/Multi-Walled Carbon Nanotube Composites. J. Dispers. Sci. Technol. 2019, 40, 94–102. [Google Scholar] [CrossRef]
- D’Avila Carvalho Erbetta, C. Synthesis and Characterization of Poly(D,L-Lactide-Co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 84965. [Google Scholar] [CrossRef]
- Xie, W.; Jiang, N.; Gan, Z. Effects of Multi-Arm Structure on Crystallization and Biodegradation of Star-Shaped Poly(ε-Caprolactone). Macromol. Biosci. 2008, 8, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Deokar, M.D.; Idage, S.B.; Idage, B.B.; Sivaram, S. Synthesis and Characterization of Well-Defined Random and Block Copolymers of ε-Caprolactone with l-Lactide as an Additive for Toughening Polylactide: Influence of the Molecular Architecture. J. Appl. Polym. Sci. 2016, 133, 43267. [Google Scholar] [CrossRef]
- Deokar, M.D.; Garnaik, B.; Sivaram, S. Toughening Poly(l-Lactide) Blends: Effectiveness of Sequence-Controlled Six-Arm Star-Branched Block Copolymers of Poly(l-Lactide) and Poly(ϵ-Caprolactone). ACS Omega 2022, 7, 9118–9129. [Google Scholar] [CrossRef]
- Doganci, M.D. Effects of Star-Shaped PCL Having Different Numbers of Arms on the Mechanical, Morphological, and Thermal Properties of PLA/PCL Blends. J. Polym. Res. 2021, 28, 1022. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, B.; Sun, B.; Sun, Z.; Xiang, S.; Li, G.; Chen, X. Preparation of High Toughness and High Transparency Polylactide Blends Resin Based on Multiarmed Polycaprolactone-Block-Poly(l-Lactide). Polym. Eng. Sci. 2016, 56, 1125–1137. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Chen, X.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P. Degradation Behavior in Vitro of Carbon Nanotubes (CNTs)/Poly(Lactic Acid) (PLA) Composite Suture. Polymers 2019, 11, 1015. [Google Scholar] [CrossRef] [Green Version]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies. Polymers 2020, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Jikei, M.; Yamadoi, Y.; Suga, T.; Matsumoto, K. Stereocomplex Formation of Poly(L-Lactide)-Poly(ε-Caprolactone) Multiblock Copolymers with Poly(D-Lactide). Polymer 2017, 123, 73–80. [Google Scholar] [CrossRef]
- Cech Barabaszová, K.; Holešová, S.; Hundáková, M.; Mohyla, V. Mechanically Treated Vermiculite Particles in PCL/Vermiculite Thin Films. Mater. Today Proc. 2022, 52, 239–247. [Google Scholar] [CrossRef]
- Luo, C.; Li, S.; Yang, M.; Xiao, W. Effect of POSS-NH2-Grafted Different Plasticizers on the Crystallization Properties of SC-Poly (l-Lactic Acid). J. Polym. Res. 2022, 29, 516. [Google Scholar] [CrossRef]
- Tie, R.; Huang, Y.; Jin, Y.; Sun, J.; Tian, H.; Lei, X.; Li, G.; Wang, L.; Men, S. Toughening Modification of Polylactic Acid by Long-Chain Hyperbranched Polymers Containing Polycaprolactone End Groups. J. Polym. Environ. 2022, 30, 5327–5338. [Google Scholar] [CrossRef]
- Mao, Y.; Talamini, B.; Anand, L. Rupture of Polymers by Chain Scission. Extrem. Mech. Lett. 2017, 13, 17–24. [Google Scholar] [CrossRef]













| Sample | Theoretical Molecular Weight | [CL]/[I] (a) | Theoretical Molar Ratio (b) | Mn | Mw | PDI |
|---|---|---|---|---|---|---|
| PLGA | 300,000 | - | 70:30 (LA:GA) | 139,871 | 302,317 | 2.161 |
| PCL-b-PDLA 5000 | 5000 | 10 | 30:70 (CL:LA) | 4713 | 5383 | 1.142 |
| PCL-b-PDLA 10,000 | 10,000 | 45 | 65:35 (CL:LA) | 10,011 | 10,873 | 1.086 |
| PCL-b-PDLA 15,000 | 15,000 | 100 | 77:23 (CL:LA) | 13,909 | 15,815 | 1.137 |
| Sample | Transmittance | |
|---|---|---|
| (At 400 nm, %) | (At 550 nm, %) | |
| PLGA (70:30) | 58.65% | 79.19% |
| PLGA/PCL-b-PDLA 10K 0.25% | 56.36% | 77.48% |
| PLGA/PCL-b-PDLA 10K 0.50% | 53.90% | 73.85% |
| PLGA/PCL-b-PDLA 10K 1.00% | 50.68% | 70.19% |
| PLGA/PCL-b-PDLA 10K 2.00% | 45.57% | 68.01% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Yoon, S.; Yang, X.; Kim, Y.J. Super-Tough and Biodegradable Poly(lactide-co-glycolide) (PLGA) Transparent Thin Films Toughened by Star-Shaped PCL-b-PDLA Plasticizers. Polymers 2023, 15, 2617. https://doi.org/10.3390/polym15122617
Jeong J, Yoon S, Yang X, Kim YJ. Super-Tough and Biodegradable Poly(lactide-co-glycolide) (PLGA) Transparent Thin Films Toughened by Star-Shaped PCL-b-PDLA Plasticizers. Polymers. 2023; 15(12):2617. https://doi.org/10.3390/polym15122617
Chicago/Turabian StyleJeong, Jieun, Sangsoo Yoon, Xin Yang, and Young Jun Kim. 2023. "Super-Tough and Biodegradable Poly(lactide-co-glycolide) (PLGA) Transparent Thin Films Toughened by Star-Shaped PCL-b-PDLA Plasticizers" Polymers 15, no. 12: 2617. https://doi.org/10.3390/polym15122617
APA StyleJeong, J., Yoon, S., Yang, X., & Kim, Y. J. (2023). Super-Tough and Biodegradable Poly(lactide-co-glycolide) (PLGA) Transparent Thin Films Toughened by Star-Shaped PCL-b-PDLA Plasticizers. Polymers, 15(12), 2617. https://doi.org/10.3390/polym15122617