Composites Based on Poly(ε-caprolactone) and Graphene Oxide Modified with Oligo/Poly(Glutamic Acid) as Biomaterials with Osteoconductive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Supplements, and Cells
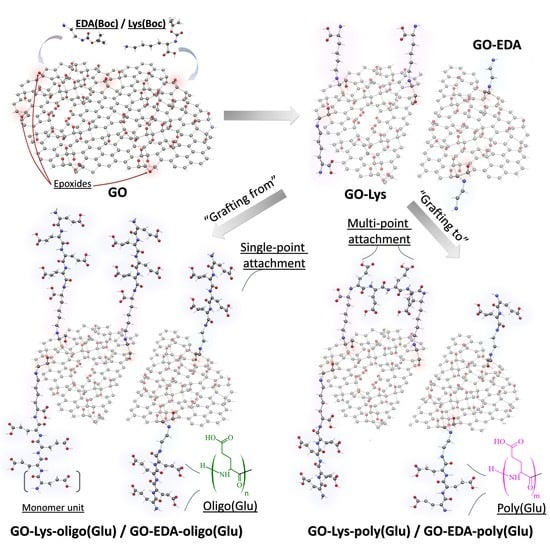
2.2. Synthesis of GO-graft-oligo(Glu) or GO-graft-poly(Glu)
2.3. Characterization of GO Derivatives
2.4. Synthesis of PCL
2.5. Manufacturing of Composite Films
2.6. Microscopic Analysis
2.7. Mechanical Properties
2.8. Cytotoxicity, Proliferation, and Biomineralization
2.8.1. Cytotoxicity
2.8.2. ALP Assay
2.8.3. Biomineralization Study
3. Results and Discussion
3.1. Modification of GO with Oligomers or Polymers of Glutamic Acid
3.2. Characterization of Modified GO-oligo(Glu)/GO-poly(Glu)
3.3. Preparation and Characterization of Composite Polymer Films
3.4. Cytotoxicity, Osteodifferentiation, and Mineralization Study
3.4.1. In Vitro Cytotoxicity and Proliferation
3.4.2. In Vitro Osteodifferentiation and Mineralization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donzelli, E.; Salvadè, A.; Mimo, P.; Viganò, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Yusof, N.M.; Idris, A. Review on nanocrystalline cellulose in bone tissue engineering applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Srouji, S.; Livne, E.; Reis, R.L.; Neves, N.M. Chitosan-poly(butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J. Tissue Eng. Regen. Med. 2012, 6, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, M.D.; Jazayeri, H.E.; Razavi, M.; Masri, R.; Tayebi, L. Three-Dimensional Bioprinting Materials with Potential Application in Preprosthetic Surgery. J. Prosthodont. 2016, 25, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-hay, A.; Barhoum, A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef]
- Castañeda-Rodríguez, S.; González-Torres, M.; Ribas-Aparicio, R.M.; Del Prado-Audelo, M.L.; Leyva-Gómez, G.; Gürer, E.S.; Sharifi-Rad, J. Recent advances in modified poly (lactic acid) as tissue engineering materials. J. Biol. Eng. 2023, 17, 21. [Google Scholar] [CrossRef]
- Zhang, H.; Hollister, S. Comparison of Bone Marrow Stromal Cell Behaviors on Poly(caprolactone) with or without Surface Modification: Studies on Cell Adhesion, Survival and Proliferation. J. Biomater. Sci. Polym. Ed. 2009, 20, 1975–1993. [Google Scholar] [CrossRef]
- Söhling, N.; Al Zoghool, S.; Schätzlein, E.; Neijhoft, J.; Costa Oliveira, K.M.; Leppik, L.; Ritz, U.; Dörsam, E.; Frank, J.; Marzi, I.; et al. In vitro Evaluation of a 20% Bioglass-Containing 3D printable PLA Composite for Bone Tissue Engineering. Int. J. Bioprinting 2022, 8, 602. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Prasad, A.; Kandasubramanian, B. Fused deposition processing polycaprolactone of composites for biomedical applications. Polym. Technol. Mater. 2019, 58, 1365–1398. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Zhang, J. Manipulating Dispersion and Distribution of Graphene in PLA through Novel Interface Engineering for Improved Conductive Properties. ACS Appl. Mater. Interfaces 2014, 6, 14069–14075. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Korzhikov-Vlakh, V.; Averianov, I.; Sinitsyna, E.; Nashchekina, Y.; Polyakov, D.; Guryanov, I.; Lavrentieva, A.; Raddatz, L.; Korzhikova-Vlakh, E.; Scheper, T.; et al. Novel Pathway for Efficient Covalent Modification of Polyester Materials of Different Design to Prepare Biomimetic Surfaces. Polymers 2018, 10, 1299. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G.; Ramirez, K.; Esquivel, M.; Jimenez, G. Biocomposite films of polylactic acid reinforced with microcrystalline cellulose from pineapple leaf fibers. J. Renew. Mater. 2019, 7, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-N.; Wang, P.-Y.G.; Wei, J.-C. Graphene Oxide-Graft-Poly(l-lactide)/Poly(l-lactide) Nanocomposites: Mechanical and Thermal Properties. Polymers 2017, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Chen, J.; Wu, D.; Chen, Y.; Lv, Q.; Wang, M. Polylactide/acetylated nanocrystalline cellulose composites prepared by a continuous route: A phase interface-property relation study. Carbohydr. Polym. 2016, 146, 58–66. [Google Scholar] [CrossRef]
- Puga, A.M.; Rey-Rico, A.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A. Hot melt poly-ε-caprolactone/poloxamine implantable matrices for sustained delivery of ciprofloxacin. Acta Biomater. 2012, 8, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, F.; Huang, Y.; Fang, Y.; Shen, M.; Zhu, M.; Shi, X. Encapsulation of Amoxicillin within Laponite-Doped Poly(lactic-co-glycolic acid) Nanofibers: Preparation, Characterization, and Antibacterial Activity. ACS Appl. Mater. Interfaces 2012, 4, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Goimil, L.; Jaeger, P.; Ardao, I.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A. Preparation and stability of dexamethasone-loaded polymeric scaffolds for bone regeneration processed by compressed CO2 foaming. J. CO2 Util. 2018, 24, 89–98. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical Applications of Graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kim, J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 10, 2001414. [Google Scholar] [CrossRef] [PubMed]
- Valapa, R.B.; Pugazhenthi, G.; Katiyar, V. Effect of graphene content on the properties of poly(lactic acid) nanocomposites. RSC Adv. 2015, 5, 28410–28423. [Google Scholar] [CrossRef]
- Camargo, J.C.; Machado, Á.R.; Almeida, E.C.; Silva, E.F.M.S. Mechanical properties of PLA-graphene filament for FDM 3D printing. Int. J. Adv. Manuf. Technol. 2019, 103, 2423–2443. [Google Scholar] [CrossRef]
- Stepanova, M.; Solomakha, O.; Rabchinskii, M.; Averianov, I.; Gofman, I.; Nashchekina, Y.; Antonov, G.; Smirnov, A.; Ber, B.; Nashchekin, A.; et al. Aminated Graphene-Graft-Oligo(Glutamic Acid) /Poly(ε-Caprolactone) Composites: Preparation, Characterization and Biological Evaluation. Polymers 2021, 13, 2628. [Google Scholar] [CrossRef]
- Pang, W.; Ni, Z.; Chen, G.; Huang, G.; Huang, H.; Zhao, Y. Mechanical and thermal properties of graphene oxide/ultrahigh molecular weight polyethylene nanocomposites. RSC Adv. 2015, 5, 63063–63072. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Graphene Oxide-Incorporated Electrospun Poly(lactic-co-glycolic acid) Nanofibrous Mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Bonderer, L.J.; Studart, A.R.; Gauckler, L.J. Bioinspired Design and Assembly of Platelet Reinforced Polymer Films. Science 2008, 319, 1069–1073. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Kaushik, A.; Arruda, E.; Waas, A.; Shim, B.; Xu, J.; Nandivada, H.; Pumplin, B.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef]
- Wei, J.; Liu, A.; Zhang, P.; Chen, L.; Chen, X.; Jing, X. The surface modification of hydroxyapatite nanoparticles by the ring opening polymerization of gamma-benzyl-l-glutamate N-carboxyanhydride. Macromol. Biosci. 2009, 9, 631–638. [Google Scholar] [CrossRef]
- Averianov, I.; Stepanova, M.; Solomakha, O.; Gofman, I.; Serdobintsev, M.; Blum, N.; Kaftuirev, A.; Baulin, I.; Nashchekina, J.; Lavrentieva, A.; et al. 3D-Printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)-modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2422–2437. [Google Scholar] [CrossRef]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Nikolaeva, A.L.; Korzhikov-Vlakh, V.A.; Karttunen, M.; Korzhikova-Vlakh, E.G. Chemical modification of nanocrystalline cellulose for improved interfacial compatibility with poly(lactic acid). Mendeleev Commun. 2019, 29, 220–222. [Google Scholar] [CrossRef]
- Gu, A.; Wu, J.; Shen, L.; Zhang, X.; Bao, N. High-Strength GO / PA66 Nanocomposite Fibers via In Situ Precipitation and Polymerization. Polymers 2021, 13, 1688. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Averianov, I.; Solomakha, O.; Zabolotnykh, N.; Gofman, I.; Serdobintsev, M.; Vinogradova, T.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Composite biomaterials based on poly(L-lactic acid) and functionalized cellulose nanocrystals. J. Renew. Mater. 2020, 8, 383–395. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials 2019, 12, 3435. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, A.; Panina, N.; Blinou, D.; Gurzhiy, V.; Nashchekina, J.; Korzhikova-Vlakh, E.; Eremin, A.; Stepanova, M. Ring-Opening Polymerization of rac-Lactide Catalyzed by Octahedral Nickel Carboxylate Complexes. Catalysts 2023, 13, 304. [Google Scholar] [CrossRef]
- Stepanova, M.; Dobrodumov, A.; Averianov, I.; Gofman, I.; Nashchekina, J.; Guryanov, I.; Klyukin, I.; Zhdanov, A.; Korzhikova-Vlakh, E.; Zhizhin, K. Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers 2022, 14, 3864. [Google Scholar] [CrossRef]
- Dzhuzha, A.Y.; Tarasenko, I.I.; Atanase, L.I.; Lavrentieva, A.; Korzhikova-Vlakh, E.G. Amphiphilic Polypeptides Obtained by the Post-Polymerization Modification of Poly(Glutamic Acid) and Their Evaluation as Delivery Systems for Hydrophobic Drugs. Int. J. Mol. Sci. 2023, 24, 1049. [Google Scholar] [CrossRef]
- de Sousa, M.; Martins, C.H.Z.; Franqui, L.S.; Fonseca, L.C.; Delite, F.S.; Lanzoni, E.M.; Martinez, D.S.T.; Alves, O.L. Covalent functionalization of graphene oxide with D-mannose: Evaluating the hemolytic effect and protein corona formation. J. Mater. Chem. B 2018, 6, 2803–2812. [Google Scholar] [CrossRef]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt electrowriting of PLA, PCL, and composite PLA/PCL scaffolds for tissue engineering application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Baptista, C.; Azagury, A.; Shin, H.; Baker, C.M.; Ly, E.; Lee, R.; Mathiowitz, E. The effect of temperature and pressure on polycaprolactone morphology. Polymer 2020, 191, 122227. [Google Scholar] [CrossRef]
- Dehnou, K.H.; Norouzi, G.S.; Majidipour, M. A review: Studying the effect of graphene nanoparticles on mechanical, physical and thermal properties of polylactic acid polymer. RSC Adv. 2023, 13, 3976–4006. [Google Scholar] [CrossRef]
- Fornes, T.; Yoon, P.; Hunter, D.; Keskkula, H.; Paul, D. Effect of organoclay structure on nylon 6 nanocomposite morphology and properties. Polymer 2002, 43, 5915–5933. [Google Scholar] [CrossRef]
- Gofman, I.V.; Abalov, I.V.; Gladchenko, S.V.; Afanas’eva, N.V. Carbon nanocones/discs—A new type of filler to improve the thermal and mechanical properties of polymer films. Polym. Adv. Technol. 2012, 23, 408–413. [Google Scholar] [CrossRef]
- Wang, C.; Lan, Y.; Yu, W.; Li, X.; Qian, Y.; Liu, H. Preparation of amino-functionalized graphene oxide/polyimide composite films with improved mechanical, thermal and hydrophobic properties. Appl. Surf. Sci. 2016, 362, 11–19. [Google Scholar] [CrossRef]
- Sahafnejad-Mohammadi, I.; Rahmati, S.; Najmoddin, N.; Bodaghi, M. Biomimetic Polycaprolactone-Graphene Oxide Composites for 3D Printing Bone Scaffolds. Macromol. Mater. Eng. 2023, 308, 2200558. [Google Scholar] [CrossRef]
- Wang, G.; He, C.; Yang, W.; Qi, F.; Qian, G.; Peng, S.; Shuai, C. Surface-Modified Graphene Oxide with Compatible Interface Enhances Poly-L-Lactic Acid Bone Scaffold. J. Nanomater. 2020, 2020, 5634096. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Rigoni, F.; Balliana, E.; Zanotti, F.; Scatto, M.; Riello, P.; Zavan, B. The Impact of Graphene Oxide on Polycaprolactone PCL Surfaces: Antimicrobial Activity and Osteogenic Differentiation of Mesenchymal Stem Cell. Coatings 2022, 12, 799. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zernik, J.; Twarog, K.; Upholt, W.B. Regulation of alkaline phosphatase and alpha2(I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation 1990, 44, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Karaman, O.; Kumar, A.; Moeinzadeh, S.; He, X.; Cui, T.; Jabbari, E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J. Tissue Eng. Regen. Med. 2016, 10, E132–E146. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Lavrentieva, A.; Korzhikov-Vlakh, V.A.; Korzhikova-Vlakh, E.G. Osteoconductive biocompatible 3D-printed composites of poly-d,l-lactide filled with nanocrystalline cellulose modified by poly(glutamic acid). Mendeleev Commun. 2022, 32, 810–812. [Google Scholar] [CrossRef]










| Sample | Conditions | Glu Content (µg/mg GO) b | Glu/Lys Ratio (mol/mol) | ||||
|---|---|---|---|---|---|---|---|
| Temperture (°C) | Reaction Time (h) | Monomer Excess a | Activation of Poly(Glu) (%) | ||||
| GO-Lys-oligo(Glu) (“grafting from”) | #1 | 35 | 48 | 1.6 | − | 7 | 4 |
| #2 | 35 | 72 | 1.6 | − | 12 | 7 | |
| #3 | 45 | 72 | 1.6 | − | 13 | 8 | |
| #4 | 45 | 72 | 4.7 | − | 19 | 12 | |
| #5 | 45 | 72 | 10 | − | 25 | 16 | |
| GO-Lys-poly(Glu) (“grafting to”) | #6 | 22 | 3 | − | 30 | 6 | 3 |
| #7 | 22 | 3 | − | 100 | 6 | 3 | |
| Sample | Grafting Conditions | DH (nm) | PDI | Zeta-Potential (mV) | |
|---|---|---|---|---|---|
| GO | − | 725 ± 33 | 0.28 | −42.9 ± 0.5 | |
| GO-Lys(Boc) | − | 1547 ± 195 | 0.40 | −42.4 ± 2.3 | |
| GO-Lys | − | 861 ± 34 | 0.43 | −34.1 ± 2.4 | |
| GO-EDA | − | 869 ± 63 | 0.36 | −37.6 ± 8.0 | |
| GO-Lys-oligo(Glu(OBzl)) | 48 h, 35 °C, ×1.6 * | 1244 ± 61 | 0.57 | −34.8 ± 5.1 | |
| GO-Lys-oligo(Glu) | #1 | 48 h, 35 °C, ×1.6 * | 518 ± 26 | 0.70 | −46.1 ± 1.4 |
| GO-Lys-oligo(Glu) | #4 | 72 h, 45 °C, ×4.7 * | 372 ± 47 | 0.65 | −42.7 ± 0.8 |
| GO-Lys-oligo(Glu) | #5 | 72 h, 45 °C, ×10 * | 465 ± 18 | 0.69 | −42.1 ± 1.7 |
| GO-Lys-poly(Glu) | #6 | 30% activation of poly(Glu) carboxyls | 672 ± 26 | 0.56 | −41.1 ± 4.1 |
| GO-Lys-poly(Glu) | #7 | 100% activation of poly(Glu) carboxyls | 898 ± 36 | 0.38 | −40.5 ± 2.3 |
| GO-EDA-oligo(Glu) | #8 | 48 h, 35 °C, ×1.6 * | 589 ± 29 | 0.43 | −38.8 ± 2.7 |
| GO-EDA-poly(Glu) | #9 | 100% activation of poly(Glu) carboxyls | 922 ± 43 | 0.33 | −40.6 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomakha, O.; Stepanova, M.; Gofman, I.; Nashchekina, Y.; Rabchinskii, M.; Nashchekin, A.; Lavrentieva, A.; Korzhikova-Vlakh, E. Composites Based on Poly(ε-caprolactone) and Graphene Oxide Modified with Oligo/Poly(Glutamic Acid) as Biomaterials with Osteoconductive Properties. Polymers 2023, 15, 2714. https://doi.org/10.3390/polym15122714
Solomakha O, Stepanova M, Gofman I, Nashchekina Y, Rabchinskii M, Nashchekin A, Lavrentieva A, Korzhikova-Vlakh E. Composites Based on Poly(ε-caprolactone) and Graphene Oxide Modified with Oligo/Poly(Glutamic Acid) as Biomaterials with Osteoconductive Properties. Polymers. 2023; 15(12):2714. https://doi.org/10.3390/polym15122714
Chicago/Turabian StyleSolomakha, Olga, Mariia Stepanova, Iosif Gofman, Yulia Nashchekina, Maxim Rabchinskii, Alexey Nashchekin, Antonina Lavrentieva, and Evgenia Korzhikova-Vlakh. 2023. "Composites Based on Poly(ε-caprolactone) and Graphene Oxide Modified with Oligo/Poly(Glutamic Acid) as Biomaterials with Osteoconductive Properties" Polymers 15, no. 12: 2714. https://doi.org/10.3390/polym15122714
APA StyleSolomakha, O., Stepanova, M., Gofman, I., Nashchekina, Y., Rabchinskii, M., Nashchekin, A., Lavrentieva, A., & Korzhikova-Vlakh, E. (2023). Composites Based on Poly(ε-caprolactone) and Graphene Oxide Modified with Oligo/Poly(Glutamic Acid) as Biomaterials with Osteoconductive Properties. Polymers, 15(12), 2714. https://doi.org/10.3390/polym15122714