Removal, Adsorption, and Cleaning of Pharmaceutical on Polyamide RO and NF Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes and Chemicals
2.2. Infrared Spectrometer with Fourier Transformation and Goniometer
2.3. Analytical Methods
2.4. Membrane Cleaning Reagents
2.5. RO/NF Laboratory System
3. Results and Discussion
3.1. Rejection Factor and Removal Mechanism of Albendazole on Used Membranes
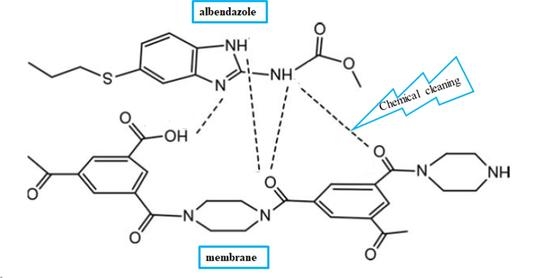
3.2. Chemical Cleaning of Adsorbed Albendazole on RO/NF Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Pena, A. Assessment of Human Pharmaceuticals in Drinking Water Catchments, Tap and Drinking Fountain Waters. Appl. Sci. 2021, 11, 7062. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Yang, Q.; Jiang, L.; Li, G. Occurrence and distribution of Pharmaceuticals and Personal Care Products (PPCPs) in wastewater related riverbank groundwater. Sci. Total Environ. 2022, 821, 153372. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Patel, H.K.; Kalaria, R.K.; Jokhakar, P.H.; Patel, C.R.; Patel, B.Y. Chapter 17—Removal of emerging contaminants in water treatment by an application of nanofiltration and reverse osmosis. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 385–400. [Google Scholar] [CrossRef]
- Rai, B.; Shrivastav, A. Chapter 26—Removal of emerging contaminants in water treatment by nanofiltration and reverse osmosis. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 605–628. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Fonseca Couto, C.; Lange, L.C.; Santos Amaral, M.C. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.D.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Kimura, K.; Toshima, S.; Amy, G.; Watanabe, Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 2004, 245, 71–78. [Google Scholar] [CrossRef]
- Zhang, H.; He, Q.; Luo, J.; Wan, Y.; Darling, S.B. Sharpening Nanofiltration: Strategies for Enhanced Membrane Selectivity. ACS Appl. Mater. Interfaces 2020, 12, 39948–39966. [Google Scholar] [CrossRef]
- Matin, A.; Jillani, S.M.S.; Baig, U.; Ihsanullah, I.; Alhooshani, K. Removal of pharmaceutically active compounds from water sources using nanofiltration and reverse osmosis membranes: Comparison of removal efficiencies and in-depth analysis of rejection mechanisms. J. Environ. Manag. 2023, 338, 117682. [Google Scholar] [CrossRef]
- Shao, L.; Cheng, X.Q.; Liu, Y.; Quan, S.; Ma, J.; Zhao, S.Z.; Wang, K.Y. Newly developed nanofiltration (NF) composite membranes by interfacial polymerization for Safranin O and Aniline blue removal. J. Membr. Sci. 2013, 430, 96–105. [Google Scholar] [CrossRef]
- Alturki, A.A.; Tadkaew, N.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010, 365, 206–215. [Google Scholar] [CrossRef]
- Awasthi, S.; Peto, R.; Read, S.; Richards, S.M.; Pande, V.; Bundy, D.; DEVTA (Deworming and Enhanced Vitamin A) Team. Population deworming every 6 months with albendazole in 1 million pre-school children in North India: DEVTA, a cluster-randomised trial. Lancet 2013, 381, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, J. Human gastrointestinal helminth infections: Are they now neglected diseases? Trends Parasitol. 2003, 19, 527–531. [Google Scholar] [CrossRef]
- Ardila, J.A.; de Alvarenga Junior, B.R.; Durango, L.C.; Soares, F.L.F.; Perlatti, B.; de Oliveira Cardoso, J.; Oliveira, R.V.; Forim, M.R.; Carneiro, R.L. Design of experiments applied to stress testing of pharmaceutical products: A case study of Albendazole. Eur. J. Pharm. Sci. 2021, 165, 105939. [Google Scholar] [CrossRef] [PubMed]
- Belew, S.; Suleman, S.; Wynendaele, E.; Duchateau, L.; De Spiegeleer, B. Environmental risk assessment of the anthelmintic albendazole in Eastern Africa, based on a systematic review. Environ. Pollut. 2021, 269, 116106. [Google Scholar] [CrossRef]
- Dolar, D.; Pelko, S.; Košutić, K.; Horvat, A.J.M. Removal of anthelmintic drugs and their photodegradation products from water with RO/NF membranes. Process Saf. Environ. Prot. 2012, 90, 147–152. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J.C. Micropollutant sorption to membrane polymers: A review of mechanisms for estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.R.D.O.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Lee, C.-H. Elucidating the Rejection Mechanisms of PPCPs by Nanofiltration and Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 6798–6806. [Google Scholar] [CrossRef]
- Couto, C.F.; Santos, A.V.; Amaral, M.C.S.; Lange, L.C.; de Andrade, L.H.; Foureaux, A.F.S.; Fernandes, B.S. Assessing potential of nanofiltration, reverse osmosis and membrane distillation drinking water treatment for pharmaceutically active compounds (PhACs) removal. J. Water Process Eng. 2020, 33, 101029. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Removal of Natural Hormones by Nanofiltration Membranes: Measurement, Modeling and Mechanisms. Environ. Sci. Technol. 2004, 38, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Braeken, L.; Ramaekers, R.; Zhang, Y.; Maes, G.; Bruggen, B.V.D.; Vandecasteele, C. Influence of hydrophobicity on retention in nanofiltration of aqueous solutions containing organic compounds. J. Membr. Sci. 2005, 252, 195–203. [Google Scholar] [CrossRef]
- Kiso, Y.; Sugiura, Y.; Kitao, T.; Nishimura, K. Effects of hydrophobicity and molecular size on rejection of aromatic pesticides with nanofiltration membranes. J. Membr. Sci. 2001, 192, 1–10. [Google Scholar] [CrossRef]
- Braeken, L.; Van der Bruggen, B.; Vandecasteele, C. Flux Decline in Nanofiltration Due to Adsorption of Dissolved Organic Compounds: Model Prediction of Time Dependency. J. Phys. Chem. B 2006, 110, 2957–2962. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Braeken, L.; Vandecasteele, C. Flux decline in nanofiltration due to adsorption of organic compounds. Sep. Purif. Technol. 2002, 29, 23–31. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.; Drewes, J.; Watanabe, Y. Adsorption of hydrophobic compounds onto NF/RO membranes: An artifact leading to overestimation of rejection. J. Membr. Sci. 2003, 221, 89–101. [Google Scholar] [CrossRef]
- Al-Amoudi, A. Effect of chemical cleaning agents on virgin nanofiltration membrane as characterized by positron annihilation spectroscopy. Sep. Purif. Technol. 2013, 110, 51–56. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Kappelhof, J.W.N.M.; Heijrnan, S.G.J.; Schippers, J.C.; van der Kooija, D. Tools for fouling diagnosis of NF and RO membranes and assessment of the fouling potential of feed water. Desalination 2003, 157, 361–365. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Roux, A.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-nitrosamine rejection by reverse osmosis: Effects of membrane exposure to chemical cleaning reagents. Desalination 2014, 343, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-J.; Kiso, Y.; Yamada, T.; Shibata, T.; Lee, T.-G. Chemical cleaning of reverse osmosis membranes used for treating wastewater from a rolling mill process. Desalination 2006, 190, 181–188. [Google Scholar] [CrossRef]
- Simon, A.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Effects of caustic cleaning on pore size of nanofiltration membranes and their rejection of trace organic chemicals. J. Membr. Sci. 2013, 447, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.; Price, W.E.; Nghiem, L.D. Effects of chemical cleaning on the nanofiltration of pharmaceutically active compounds (PhACs). Sep. Purif. Technol. 2012, 88, 208–215. [Google Scholar] [CrossRef]
- Simon, A.; Price, W.E.; Nghiem, L.D. Influence of formulated chemical cleaning reagents on the surface properties and separation efficiency of nanofiltrationmembranes. J. Membr. Sci. 2013, 432, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.; Price, W.E.; Nghiem, L.D. Changes in surface properties and separation efficiency of a nanofiltration membrane after repeated fouling and chemical cleaning cycles. Sep. Purif. Technol. 2013, 113, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.; Price, W.E.; Nghiem, L.D. Impact of chemical cleaning on the nanofiltration of pharmaceutically active compounds (PhACs): The role of cleaning temperature. J. Taiwan Inst. Chem. Eng. 2013, 44, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Dolar, D.; Drašinac, N.; Košutić, K.; Škorić, I.; Ašperger, D. Adsorption of hydrophilic and hydrophobic pharmaceuticals on RO/NF membranes: Identification of interactions using FTIR. J. Appl. Polym. Sci. 2017, 134, 44426. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dolar, D.; Vuković, A.; Ašperger, D.; Košutić, K. Effect of water matrices on removal of veterinary pharmaceuticals by nanofiltration and reverse osmosis membranes. J. Environ. Sci. 2011, 23, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Castaño Osorio, S.; Biesheuvel, P.M.; Spruijt, E.; Dykstra, J.E.; van der Wal, A. Modeling micropollutant removal by nanofiltration and reverse osmosis membranes: Considerations and challenges. Water Res. 2022, 225, 119130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Wang, X.-M.; Yang, H.-W.; Xie, Y.F. Adsorption of pharmaceuticals onto isolated polyamide active layer of NF/RO membranes. Chemosphere 2018, 200, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Wang, X.-M.; Yang, H.-W.; Xie, Y.F. Quantifying the influence of solute-membrane interactions on adsorption and rejection of pharmaceuticals by NF/RO membranes. J. Membr. Sci. 2018, 551, 37–46. [Google Scholar] [CrossRef]
- Dolar, D.; Košutić, K.; Ašperger, D. Influence of adsorption of pharmaceuticals onto RO/NF membranes on their removal from water. Water Air Soil Pollut. 2013, 224, 1–13. [Google Scholar] [CrossRef]
- Akin, O.; Temelli, F. Probing the hydrophobicity of commercial reverse osmosis membranes produced by interfacial polymerization using contact angle, XPS, FTIR, FE-SEM and AFM. Desalination 2011, 278, 387–396. [Google Scholar] [CrossRef]
- Torrado, S.; Torrado, S.; Cadorniga, R.; Torrado, J.J. Formulation parameters of albendazole solution. Int. J. Pharm. 1996, 140, 45–50. [Google Scholar] [CrossRef]






| Molecular Structure | Direction of Dipole Moment | ||
|---|---|---|---|
| CAS number | 54965-21-8 |  |  |
| Molecular weight, Mw (g/mol) | 265.33 | ||
| Water solubility (mg/L) 1 | 46.39 | ||
| width (nm) 2 | 0.482 | ||
| height (nm) 2 | 0.279 | ||
| length (nm) 2 | 1.632 | ||
| log KO/W | 3.07 | ||
| log D (pH = 7.4) 3 | 3.06 | ||
| pKa 4 | 6.90 | ||
| Dipole moment, μ (D) 5 | 4.33 | ||
| Charge at pH 7 | negative |
| MWCO t/h | UTC-70HA 100 | XLE 100 | BW30 100 | NF90 100 | NF 150–300 | NF270 150–300 |
|---|---|---|---|---|---|---|
| R/% | ||||||
| 0 | 78.1 | 78.6 | 78.5 | 79.1 | 73.8 | 65.4 |
| 4 | 61.2 | 63.7 | 62.8 | 62.4 | 44.8 | 43.8 |
| γp/mg/L | ||||||
| 0 | 3.39 | 3.32 | 3.33 | 3.24 | 4.06 | 3.24 |
| 4 | 3.91 | 3.66 | 3.75 | 3.79 | 5.56 | 3.79 |
| t/h | γF/mg/L |
|---|---|
| 0 | 15.50 |
| 1 | 12.76 |
| 2 | 12.41 |
| 3 | 11.96 |
| 4 | 10.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolar, D.; Ćurić, I.; Ašperger, D. Removal, Adsorption, and Cleaning of Pharmaceutical on Polyamide RO and NF Membranes. Polymers 2023, 15, 2745. https://doi.org/10.3390/polym15122745
Dolar D, Ćurić I, Ašperger D. Removal, Adsorption, and Cleaning of Pharmaceutical on Polyamide RO and NF Membranes. Polymers. 2023; 15(12):2745. https://doi.org/10.3390/polym15122745
Chicago/Turabian StyleDolar, Davor, Iva Ćurić, and Danijela Ašperger. 2023. "Removal, Adsorption, and Cleaning of Pharmaceutical on Polyamide RO and NF Membranes" Polymers 15, no. 12: 2745. https://doi.org/10.3390/polym15122745
APA StyleDolar, D., Ćurić, I., & Ašperger, D. (2023). Removal, Adsorption, and Cleaning of Pharmaceutical on Polyamide RO and NF Membranes. Polymers, 15(12), 2745. https://doi.org/10.3390/polym15122745