Polylactic Acid/Lignin Composites: A Review
Abstract
:1. Introduction
1.1. Poly (Lactic Acid)
1.2. Lignin
2. Research Progress of Polylactic Acid-Lignin Composites
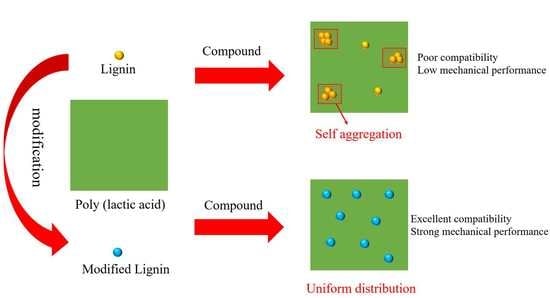
2.1. Physical Blending
2.2. Chemical Modification
2.2.1. Esterification Modification of Lignin
2.2.2. Acetylation Modification of Lignin
2.2.3. Modification of Lignin by Graft Copolymerization
Ordinary Free Radical Polymerization
Ring-Opening Polymerization
2.2.4. Further Options of Lignin Modification
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liu, S.; Wang, Q.; Ji, X.; Yang, G.; Chen, J.; Fatehi, P. Strong, ductile and biodegradable polylactic acid/lignin-containing cellulose nanofibril composites with improved thermal and barrier properties. Ind. Crops Prod. 2021, 171, 113898. [Google Scholar] [CrossRef]
- Mahmud, S.; Long, Y.; Abu Taher, M.; Xiong, Z.; Zhang, R.; Zhu, J. Toughening polylactide by direct blending of cellulose nanocrystals and epoxidized soybean oil. J. Appl. Polym. Sci. 2019, 136, 48221. [Google Scholar] [CrossRef]
- Gu, Q.; Eberhardt, T.L.; Shao, J.; Pan, H. Preparation of an oxyalkylated lignin-g-polylactic acid copolymer to improve the compatibility of an organosolv lignin in blended poly(lactic acid) films. J. Appl. Polym. Sci. 2022, 139, 52003. [Google Scholar] [CrossRef]
- Fournier, L.; Rivera Mirabal, D.M.; Hillmyer, M.A. Toward Sustainable Elastomers from the Grafting-Through Polymerization of Lactone-Containing Polyester Macromonomers. Macromolecules 2022, 55, 1003–1014. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties-from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.S.; Lee, S.-Y.; Mahajan, R.L.; Kim, Y.-T. Exploration of hybrid nanocarbon composite with polylactic acid for packaging applications. Int. J. Biol. Macromol. 2020, 144, 135–142. [Google Scholar] [CrossRef]
- Li, R.; Wu, L.; Li, B.-G. Poly(L-lactide) Materials with Balanced Mechanical Properties Prepared by Blending with PEG-mb-PPA Multiblock Copolymers. Ind. Eng. Chem. Res. 2017, 56, 2773–2782. [Google Scholar] [CrossRef]
- Ma, H.; Shen, J.; Yang, Q.; Zhou, J.; Xia, S.; Cao, J. Effect of the Introduction of Fish Collagen on the Thermal and Mechanical Properties of Poly(lactic acid). Ind. Eng. Chem. Res. 2015, 54, 10945–10951. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly(lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Harynska, A.; Janik, H.; Sienkiewicz, M.; Mikolaszek, B.; Kucinska-Lipka, J. PLA-Potato Thermoplastic Starch Filament as a Sustainable Alternative to the Conventional PLA Filament: Processing, Characterization, and FFF 3D Printing. ACS Sustain. Chem. Eng. 2021, 9, 6923–6938. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (PLA) biocomposite: Processing, additive manufacturing and advanced applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Q.; Li, X.; Meng, Y.; Ling, Z.; Ji, Z.; Chen, F. Preparation and Characterization of Degradable Cellulose-Based Paper with Superhydrophobic, Antibacterial, and Barrier Properties for Food Packaging. Int. J. Mol. Sci. 2022, 23, 11158. [Google Scholar] [CrossRef]
- Roy Goswami, S.; Sudhakaran Nair, S.; Zhang, X.; Tanguy, N.; Yan, N. Starch Maleate/Epoxidized Soybean Oil/Polylactic Acid Films with Improved Ductility and Biodegradation Potential for Packaging Fatty Foods. ACS Sustain. Chem. Eng. 2022, 10, 14185–14194. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Maciel Filho, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Valente, T.A.M.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Effect of Sterilization Methods on Electrospun Poly(lactic acid) (PLA) Fiber Alignment for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 3241–3249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, H.; Yuan, M.; Yuan, M.; Chen, H. Poly(lactic acid) blends with poly(trimethylene carbonate) as biodegradable medical adhesive material. Int. J. Mol. Sci. 2017, 18, 2041. [Google Scholar] [CrossRef] [Green Version]
- Hollingshead, S.; Liu, J.C.; Siebert, H.; Wilker, J.J.; Wilker, J.J.; Liu, J.C. Cytocompatibility of a mussel-inspired poly(lactic acid)-based adhesive. J. Biomed. Mater. Res. A 2022, 110, 43–51. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of biodegradable polylactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Hassan, A.; Imran, M.; Wahit, M.U. Toughening of Polylactic Acid Nanocomposites: A Short Review. Polym.-Plast. Technol. Eng. 2012, 51, 175–192. [Google Scholar] [CrossRef]
- Zolali, A.M.; Favis, B.D. Toughening of Cocontinuous Polylactide/Polyethylene Blends via an Interfacially Percolated Intermediate Phase. Macromolecules 2018, 51, 3572–3581. [Google Scholar] [CrossRef]
- Zhang, T.; Han, W.; Zhang, C.; Weng, Y. Effect of chain extender and light stabilizer on the weathering resistance of PBAT/PLA blend films prepared by extrusion blowing. Polym. Degrad. Stab. 2021, 183, 109455. [Google Scholar] [CrossRef]
- Gu, L.; Macosko, C.W. Evaluating PE/PLA interfacial tension using ternary immiscible polymer blends. J. Appl. Polym. Sci. 2021, 138, 50623. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Beaucamp, A.; Muddasar, M.; Amiinu, I.S.; Moraes Leite, M.; Culebras, M.; Latha, K.; Gutierrez, M.C.; Rodriguez-Padron, D.; del Monte, F.; Kennedy, T.; et al. Lignin for energy applications—state of the art, life cycle, technoeconomic analysis and future trends. Green Chem. 2022, 24, 8193–8226. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Jedrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, L. The role of lignin and lignin-based materials in sustainable construction-A comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alen, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. BioResources 2019, 14, 1–52. [Google Scholar] [CrossRef]
- Kienberger, M.; Maitz, S.; Pichler, T.; Demmelmayer, P. Systematic Review on Isolation Processes for Technical Lignin. Processes 2021, 9, 804. [Google Scholar] [CrossRef]
- Naron, D.R.; Collard, F.X.; Tyhoda, L.; Gorgens, J.F. Characterisation of lignins from different sources by appropriate analytical methods: Introducing thermogravimetric analysis-thermal desorption-gas chromatography-mass spectroscopy. Ind. Crops Prod. 2017, 101, 61–74. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; Serrano, L.; Labidi, J. Lignin purification with green solvents. Cellul. Chem. Technol. 2012, 46, 221–225. [Google Scholar]
- Wang, H.; Chen, X.; Zhang, L.; Li, Z.; Fan, X.; Sun, S. Efficient production of lignin-based slow-release nitrogen fertilizer via microwave heating. Ind. Crops Prod. 2021, 166, 113481. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A simple and effective approach to fabricate lignin nanoparticles with tunable sizes based on lignin fractionation. Green Chem. 2020, 22, 2011–2017. [Google Scholar] [CrossRef]
- Thielemans, W.; Can, E.; Morye, S.S.; Wool, R.P. Novel applications of lignin in composite materials. J. Appl. Polym. Sci. 2002, 83, 323–331. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of polylactic acid composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Zhai, S.; Liu, Q.; Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. A Review: Research Progress in Modification of Poly(Lactic Acid) by Lignin and Cellulose. Polymers 2021, 13, 776. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Foulon, L.; Chabbert, B.; Aguie-Beghin, V. Natural Organic UV-Absorbent Coatings Based on Cellulose and Lignin: Designed Effects on Spectroscopic Properties. Biomacromolecules 2012, 13, 4081–4088. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Naguib, H.E.; Celzard, A.; Fierro, V. Comparison of the thermal, dynamic mechanical and morphological properties of PLA-lignin & PLA-tannin particulate green composites. Compos. Part B 2015, 82, 92–99. [Google Scholar] [CrossRef]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef]
- Pawale, S.; Kalia, K.; Alshammari, S.; Cronin, D.; Zhang, X.; Ameli, A. Deep Eutectic Solvent-Extracted Lignin as an Efficient Additive for Entirely Biobased Polylactic Acid Composites. ACS Appl. Polym. Mater. 2022, 4, 5858–5868. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of processing conditions and lignin content on thermal, mechanical and degradative behavior of lignin nanoparticles/polylactic (acid) bionanocomposites prepared by melt extrusion and solvent casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV protective, antioxidant, antibacterial and compostable polylactic acid composites containing pristine and chemically modified lignin nanoparticles. Molecules 2021, 26, 126. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Egues, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egues, I.; Labidi, J. Kraft lignin as filler in PLA to improve ductility and thermal properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Kumar, A.; Tumu, V.R.; Ray Chowdhury, S.R.; Reddy S.V.S., R. A green physical approach to compatibilize a bio-based poly(lactic acid)/lignin blend for better mechanical, thermal and degradation properties. Int. J. Biol. Macromol. 2019, 121, 588–600. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Thunga, M.; Chen, K.; Grewell, D.; Kessler, M.R. Bio-renewable precursor fibers from lignin/polylactide blends for conversion to carbon fibers. Carbon 2014, 68, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Park, C.-W.; Han, S.-Y.; Bandi, R.; Dadigala, R.; Lee, E.-A.; Kim, J.-K.; Cindradewi, A.W.; Kwon, G.-J.; Lee, S.-H. Esterification of Lignin Isolated by Deep Eutectic Solvent Using Fatty Acid Chloride, and Its Composite Film with Poly(lactic acid). Polymers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of chemically modified lignin-reinforced PLA biocomposites and their 3D printing performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Zhao, X.; Zhai, S.; Wang, Q.; Li, C.; Zhang, X. Utilization of lignin upon successive fractionation and esterification in polylactic acid (PLA)/lignin biocomposite. Int. J. Biol. Macromol. 2022, 203, 49–57. [Google Scholar] [CrossRef]
- Kim, Y.J.; Suhr, J.H.; Seo, H.W.; Sun, H.N.; Kim, S.H.; Park, I.K.; Kim, S.H.; Lee, Y.K.; Kim, K.J.; Nam, J.D. All Biomass and UV Protective Composite Composed of Compatibilized Lignin and Poly (Lactic-acid). Sci. Rep. 2017, 7, 43596. [Google Scholar] [CrossRef] [Green Version]
- Esakkimuthu, E.S.; DeVallance, D.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional lignin-poly (lactic acid) biocomposites for packaging applications. Front. Bioeng. Biotechnol. 2022, 10, 1025076. [Google Scholar] [CrossRef]
- Ge, X.; Chang, M.; Jiang, W.; Zhang, B.; Xing, R.; Bulin, C. Investigation on two modification strategies for the reinforcement of biodegradable lignin/poly(lactic acid) blends. J. Appl. Polym. Sci. 2020, 137, 49354. [Google Scholar] [CrossRef]
- Zong, E.; Liu, X.; Liu, L.; Wang, J.; Song, P.; Ma, Z.; Ding, J.; Fu, S. Graft Polymerization of Acrylic Monomers onto Lignin with CaCl2-H2O2 as Initiator: Preparation, Mechanism, Characterization, and Application in Poly(lactic acid). ACS Sustain. Chem. Eng. 2018, 6, 337–348. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(lactic acid) Composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A Renewable Lignin-Lactide Copolymer and Application in Biobased Composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Boarino, A.; Schreier, A.; Leterrier, Y.; Klok, H.-A. Uniformly Dispersed Poly(lactic acid)-Grafted Lignin Nanoparticles Enhance Antioxidant Activity and UV-Barrier Properties of Poly(lactic acid) Packaging Films. ACS Appl. Polym. Mater. 2022, 4, 4808–4817. [Google Scholar] [CrossRef]
- Wu, M.; Wu, M.; Pan, M.; Jiang, F.; Hui, B.; Zhou, L. Synthesization and Characterization of Lignin-graft-Poly (Lauryl Methacrylate) via ARGET ATRP. Int. J. Biol. Macromol. 2022, 207, 522–530. [Google Scholar] [CrossRef]
- Bao, X.; Yu, Y.; Wang, Q.; Wang, P.; Yuan, J. “Graft to” Modification of Lignin by the Combination of Enzyme-Initiated Reversible Addition-Fragmentation Chain Transfer and Grafting. ACS Sustain. Chem. Eng. 2019, 7, 12973–12980. [Google Scholar] [CrossRef]
- Wang, K.; Bauer, S.; Sun, R.-c. Structural Transformation of Miscanthus × giganteus Lignin Fractionated under Mild Formosolv, Basic Organosolv, and Cellulolytic Enzyme Conditions. J. Agric. Food Chem. 2012, 60, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Galeski, A.; Pawlak, A. PBAT green composites: Effects of kraft lignin particles on the morphological, thermal, crystalline, macro and micromechanical properties. Polymer 2020, 203, 122748. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.R.; Fatehi, P. Water soluble kraft lignin-acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Zhang, Z.; Mulyadi, A.; Kuang, X.; Liu, W.; Li, V.; Gogoi, P.; Liu, X.; Deng, Y. Lignin-polystyrene composite foams through high internal phase emulsion polymerization. Polym. Eng. Sci. 2019, 59, 964–972. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, L.P.; Lu, X.H.; He, C.B. Biodegradable and renewable poly(lactide)-lignin composites: Synthesis, interface and toughening mechanism. J. Mater. Chem. A 2015, 3, 3699–3709. [Google Scholar] [CrossRef]
- Qian, M.; Sun, Y.; Xu, X.; Liu, L.; Song, P.; Yu, Y.; Wang, H.; Qian, J. 2D-alumina platelets enhance mechanical and abrasion properties of PA612 via interfacial hydrogen-bond interactions. Chem. Eng. J. 2017, 308, 760–771. [Google Scholar] [CrossRef]
- Gupta, C.; Washburn, N.R. Polymer-Grafted Lignin Surfactants Prepared via Reversible Addition-Fragmentation Chain-Transfer Polymerization. Langmuir 2014, 30, 9303–9312. [Google Scholar] [CrossRef]
- Huang, W.; Wu, M.; Liu, W.; Hua, Z.; Wang, Z.; Zhou, L. Value-adding of organosolv lignin: Designing mechanically robust UV-resistant polymeric glass via ARGET ATRP. Appl. Surf. Sci. 2019, 475, 302–311. [Google Scholar] [CrossRef]









| Extraction Method | Type of Lignin | Scale | Reaction Condition | Solubility | Ref. |
|---|---|---|---|---|---|
| Sulfur-containing | Kraft lignin | Industrial | 170 °C, NaOH + Na2S | Alkali, organic solvents | [29] |
| Lignosulfonate | Industrial | 140 °C, SO2+Na+/Ca+/Mg+, NH4+ | Water | [30] | |
| Sulfur-free | Soda lignin | Industrial/Pilot | 150–170 °C, NaOH | Alkali | [31] |
| Organosolv lignin | Industrial/Pilot | 90–210 °C, Organosolv | Wide range of organic solvents | [32] |
| PLA | Type of LG * | LG Mass Fraction (%) | TS ** (MPa) | EB *** (%) | Ref. |
|---|---|---|---|---|---|
| 3052D | KL | 0 | 70.16 | - | [39] |
| 3052D | KL | 15 | 55.24 | - | [39] |
| 2002D | LO | 0 | 58.76 | 6.00 | [40] |
| 2002D | LO | 7 | 48.76 | 2.50 | [40] |
| 3052D | CAL | 0 | 66.31 | 4.34 | [41] |
| 3052D | CAL | 10 | 11.88 | 0.90 | [41] |
| 3251D | LNPs | 0 | 44.30 | 16.80 | [42] |
| 3251D | LNPs | 1 | 48.70 | 26.70 | [42] |
| 3251D | LNP | 0 | 49.92 | 8.23 | [43] |
| 3251D | LNP | 3 | 53.65 | 3.35 | [43] |
| Modification Method * | Reaction Monomer ** | Reaction Condition *** | Ref. |
|---|---|---|---|
| Esterification | Lignin, PC | THF, 65 °C, 48 h, N2 | [50] |
| Esterification | Lignin, MA | DMF, 120 °C, 6 h | [51] |
| Esterification | Lignin, SAn | THF, 60 °C, 2 h | [52] |
| Acetylation | Lignin, AA, Py | Formamide, RT, 36 h | [45] |
| Acetylation | Lignin, AA, Py | RT, 24 h | [53] |
| Hydroxypropylation | Lignin, PPC | NaOH, 170 °C, 3 h | [54] |
| FRP | Lignin, MA | Melting compound | [55] |
| FRP | Lignin, MMA, BMA | DMSO, 50 °C, 24 h | [56] |
| FRP | Lignin, LMA, THFMA | DMSO, 50 °C, 24 h | [57] |
| ROP | Lignin, L-lactide | 130 °C, 3.5 h, N2 | [58] |
| ROP | LNP, DL-lactide | RT, 3 h, N2 | [59] |
| ATRP | 1. Lignin, TEA, BiBB 2. LMA, Bpy, CuBr2 | 1. THF, 65 °C, 48 h, N2 2. THF,70 °C, 24 h, N2 | [60] |
| RAFT | Lignin, DDMAT, HRP | Buffer (0.1 M, pH 7.0) 40 °C, 24 h | [61] |
| Sample * | TS (MPa) | EM ** (GPa) | EB (%) | Ref. |
|---|---|---|---|---|
| PLA/10%LG | 36.6 | 0.26 | 1.3 | [50] |
| PLA/10%ELG | 42.3 | 0.31 | 1.1 | [50] |
| PLA/20%LG | 23 | 2.4 | - | [51] |
| PLA/20%ELG | 55 | 5.0 | - | [51] |
| PLA/5%LG | 43.5 | 0.22 | 1.9 | [45] |
| PLA/5%ALG | 44.5 | 0.26 | 3.9 | [45] |
| PLA/5%LG | 65.2 | 2.53 | 7.9 | [53] |
| PLA/5%ALG | 70.3 | 2.51 | 9.5 | [53] |
| PLA/5%LG | 33.6 | - | 4.6 | [54] |
| PLA/5%OPKL | 39.6 | - | 24.6 | [54] |
| PLA/4%LG | 59.9 | 3.2 | 3.8 | [55] |
| PLA/4%LG/0.4%DCP | 64.2 | 3.1 | 3.6 | [55] |
| PLA–LG/0.4%MA | 71.6 | 5.0 | 4.2 | [55] |
| PLA/10%LG | 59.2 | 1.1 | 6.7 | [56] |
| PLA/10%LG-g-PBMA | 61.5 | 0.9 | 28 | [56] |
| PLA | 63.5 | 2.4 | 12 | [57] |
| PLA/20%MLG | 40.9 | 2.46 | 204 | [57] |
| PLA/10%LG | 24.9 | 2.8 | 1.1 | [59] |
| PLA/10%PLA-g-LNPs | 23.5 | 1.9 | 8.5 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Liu, G.; Sun, H.; Weng, Y. Polylactic Acid/Lignin Composites: A Review. Polymers 2023, 15, 2807. https://doi.org/10.3390/polym15132807
Shi K, Liu G, Sun H, Weng Y. Polylactic Acid/Lignin Composites: A Review. Polymers. 2023; 15(13):2807. https://doi.org/10.3390/polym15132807
Chicago/Turabian StyleShi, Kang, Guoshuai Liu, Hui Sun, and Yunxuan Weng. 2023. "Polylactic Acid/Lignin Composites: A Review" Polymers 15, no. 13: 2807. https://doi.org/10.3390/polym15132807
APA StyleShi, K., Liu, G., Sun, H., & Weng, Y. (2023). Polylactic Acid/Lignin Composites: A Review. Polymers, 15(13), 2807. https://doi.org/10.3390/polym15132807