Design of a 3D Amino-Functionalized Rice Husk Ash Nano-Silica/Chitosan/Alginate Composite as Support for Laccase Immobilization
Abstract
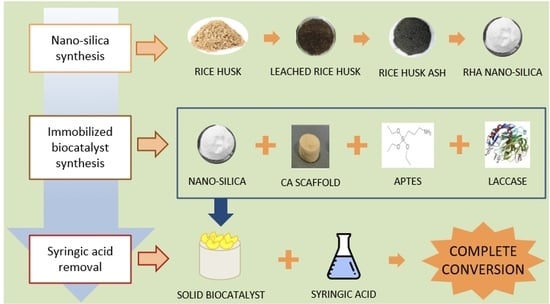
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification and Calcination of Rice Husk
2.3. Silica Extraction from Rice Husk Ash
2.4. Chitosan/Alginate (CA) Scaffold Preparation
2.5. RHA Nano-Silica/Chitosan/Alginate (RCA) Scaffold Preparation
2.6. Functionalization of RHA Nano-Silica/Chitosan/Alginate (RCA) Scaffold with APTES
2.7. Samples Characterization
2.7.1. Elemental and ATR-FTIR Spectroscopy Analysis
2.7.2. Scanning Electron Microscopy (SEM) Analysis
2.7.3. Powder X-ray Diffraction (XRD), BET Analysis, and Porosity Determination
2.7.4. Mechanical Testing and Thermal Stability of the Scaffolds
2.8. Laccase Immobilization on APTES/RHA Nano-Silica/Chitosan/Alginate (ARCA) Scaffold
2.9. Free and Immobilized Activity Determination
2.10. Evaluation of the Immobilized Biocatalysts
2.11. Operational Stability and Syringic Acid Oxidation
3. Results and Discussion
3.1. Characterization of RHA Nano-Silica
3.2. Characterization of RHA Nano-Silica/Chitosan/Alginate (RCA) Scaffold
3.3. Functionalization of RHA Nano-Silica/Chitosan/Alginate Scaffold with APTES
3.4. Immobilization of Laccase on Silica Functionalized Scaffold
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Prata, A.S.; Forte, M.B.S. Enzyme immobilization: What have we learned in the past five years? Biofuels Bioprod. Biorefining 2021, 16, 587–608. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in Food Industry: Bioprocessing, Potential Industrial and Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, S.R. Dye removal by immobilised fungi. Biotechnol. Adv. 2009, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sonal, M.; Nidhi, P.; Rajput, K.; Rakeshkumar, P. A Review: Properties, Mode of Action and Applications of Fungal Laccase. Int. Res. J. Eng. Technol. 2020, 7, 42–48. [Google Scholar]
- Al-Sareji, O.J.; Meiczinger, M.; Salman, J.M.; Al-Juboori, R.A.; Hashim, K.S.; Somogyi, V.; Jakab, M. Ketoprofen and aspirin removal by laccase immobilized on date stones. Chemosphere 2023, 311, 137133. [Google Scholar] [CrossRef]
- Sharma, K.; Tewatia, P.; Kaur, M.; Pathania, D.; Banat, F.; Rattan, G.; Singhal, S.; Kaushik, A. Bioremediation of multifarious pollutants using laccase immobilized on magnetized and carbonyldiimidazole-functionalized cellulose nanofibers. Sci. Total Environ. 2023, 864, 161137. [Google Scholar] [CrossRef]
- Chen, Z.; Oh, W.-D.; Yap, P.-S. Recent advances in the utilization of immobilized laccase for the degradation of phenolic compounds in aqueous solutions: A review. Chemosphere 2022, 307, 135824. [Google Scholar] [CrossRef]
- Apriceno, A.; Silvestro, I.; Girelli, A.; Francolini, I.; Pietrelli, L.; Piozzi, A. Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation. Polymers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Lau, C.; Windmeisser, M.; Liaw, B.Y.; Klotzbach, T.; Minteer, S.D. Design of chitosan gel pore structure: Towards enzyme catalyzed flow-through electrodes. J. Mater. Chem. 2008, 18, 667–674. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Peksel, A. Fabrication of PVA-chitosan-based nanofibers for phytase immobilization to enhance enzymatic activity. Int. J. Biol. Macromol. 2020, 164, 3315–3322. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2019, 18, 315–323. [Google Scholar] [CrossRef]
- Monteiro, O.A.; Airoldi, C. Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.; Utturkar, G.; Haggard, W.; Yang, Y.; Ong, J.; Bumgardner, J. The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohydr. Polym. 2007, 68, 561–567. [Google Scholar] [CrossRef]
- Bernabé, P.; Peniche, C.; Argüelles-Monal, W. Swelling behavior of chitosan/pectin polyelectrolyte complex membranes. Effect of thermal cross-linking. Polym. Bull. 2005, 55, 367–375. [Google Scholar] [CrossRef]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolyte complex formation between alginate and chitosan as a function of pH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Gupta, M.; Raghava, S. Smart systems based on polysaccharides. In Natural-Based Polymers for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2008; pp. 129–161. [Google Scholar] [CrossRef]
- Kopplin, G.; Lervik, A.; Draget, K.I.; Aachmann, F.L. Alginate gels crosslinked with chitosan oligomers—A systematic investigation into alginate block structure and chitosan oligomer interaction. RSC Adv. 2021, 11, 13780–13798. [Google Scholar] [CrossRef]
- Mustafa, A.; Tomescu, A.; Mustafa, E.; Cherim, M.; Sîrbu, R. Polyelectrolyte Complexes Based on Chitosan and Natural Polymers. Eur. J. Interdiscip. Stud. 2016, 4, 100–107. [Google Scholar] [CrossRef]
- Praveenkumara, J.; Madhu, P.; Gowda, T.G.Y.; Sanjay, M.R.; Siengchin, S. A comprehensive review on the effect of synthetic filler materials on fiber-reinforced hybrid polymer composites. J. Text. Inst. 2021, 113, 1231–1239. [Google Scholar] [CrossRef]
- Zhuang, C.; Chen, Y. The effect of nano-SiO2 on concrete properties: A review. Nanotechnol. Rev. 2019, 8, 562–572. [Google Scholar] [CrossRef] [Green Version]
- Joglekar, S.N.; Kharkar, R.A.; Mandavgane, S.A.; Kulkarni, B.D. Process development of silica extraction from RHA: A cradle to gate environmental impact approach. Environ. Sci. Pollut. Res. 2018, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Bazzi, L.; Hesemann, P.; Laassiri, S.; EL Hankari, S. Alternative approaches for the synthesis of nano silica particles and their hybrid composites: Synthesis, properties, and applications. Int. J. Environ. Sci. Technol. 2023, 1–40. [Google Scholar] [CrossRef]
- Nayak, P.P.; Nandi, S.; Datta, A.K. Comparative assessment of chemical treatments on extraction potential of commercial grade silica from rice husk. Eng. Rep. 2019, 1, e12035. [Google Scholar] [CrossRef] [Green Version]
- Pode, R. Potential applications of rice husk ash waste from rice husk biomass power plant. Renew. Sustain. Energy Rev. 2016, 53, 1468–1485. [Google Scholar] [CrossRef]
- Girelli, A.M.; Chiappini, V. Renewable, sustainable, and natural lignocellulosic carriers for lipase immobilization: A review. J. Biotechnol. 2023, 365, 29–47. [Google Scholar] [CrossRef]
- Hossain, S.S.; Mathur, L.; Roy, P.K. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Ajeel, S.A.; Sukkar, K.A.; Zedin, N.K. Evaluation of acid leaching process and calcination temperature on the silica extraction efficiency from the sustainable sources. J. Phys. Conf. Ser. 2021, 1773, 012014. [Google Scholar] [CrossRef]
- Prasad, R.; Pandey, M. Rice Husk Ash as a Renewable Source for the Production of Value Added Silica Gel and its Application: An Overview. Bull. Chem. React. Eng. Catal. 2012, 7, 1–25. [Google Scholar] [CrossRef]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Azat, S.; Korobeinyk, A.V.; Moustakas, K.; Inglezakis, V.J. Sustainable production of pure silica from rice husk waste in Kazakhstan. J. Clean. Prod. 2019, 217, 352–359. [Google Scholar] [CrossRef]
- Nayak, P.; Datta, A. Synthesis of SiO2-Nanoparticles from Rice Husk Ash and its Comparison with Commercial Amorphous Silica through Material Characterization. Silicon 2020, 13, 1209–1214. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, T.-J.; Gao, H.; Jin, Y. High density silanization of nano-silica particles using γ-aminopropyltriethoxysilane (APTES). Appl. Surf. Sci. 2015, 351, 646–654. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Amy, S.R.; Chabal, Y.J. Attachment of 3-(Aminopropyl)triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. Review: 3-Aminopropyltriethoxysilane (APTES) Deposition Methods on Oxide Surfaces in Solution and Vapor Phases for Biosensing Applications. Biosensors 2022, 13, 36. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; Sapag, K. Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Microporous Mesoporous Mater. 2014, 200, 68–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z.; Yin, R.; Yang, D.; Nie, J. Alginate–chitosan/hydroxyapatite polyelectrolyte complex porous scaffolds: Preparation and characterization. Int. J. Biol. Macromol. 2010, 46, 199–205. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process. Biochem. 2019, 90, 66–80. [Google Scholar] [CrossRef]
- Dashan, E.; Khadar, M. Evaluating the Properties of Acha Husk Ash. Int. J. Sci. Eng. Technol. 2011, 32, 167–171. [Google Scholar]
- Abu Bakar, R.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, E.; Shahebrahimi, S.; Feyzi, M.; Shaterzadeh, M. Optimization of synthesis and characterization of nanosilica produced from rice husk (a common waste material). Int. Nano Lett. 2012, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Venkateswaran, S.; Yuvakkumar, R.; Rajendran, V. Nano Silicon from Nano Silica Using Natural Resource (Rha) for Solar Cell Fabrication. Phosphorus Sulfur Silicon Relat. Elements 2013, 188, 1178–1193. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Sánchez, F.A.L.; Camacho, A.L.D.; Rocha, T.L.A.D.C.; Moraes, C.A.M.; Sousa, V. Characterization of Silica Produced from Rice Husk Ash: Comparison of Purification and Processing Methods. Mater. Res. 2017, 20, 512–518. [Google Scholar] [CrossRef]
- Kara, F.; Demirel, G.; Tümtürk, H. Immobilization of urease by using chitosan–alginate and poly(acrylamide-co-acrylic acid)/κ-carrageenan supports. Bioprocess Biosyst. Eng. 2006, 29, 207–211. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, J.; Zhao, J.; Chen, S.; Yan, X. Regulating Preparation Of Functional Alginate-Chitosan Three-Dimensional Scaffold For Skin Tissue Engineering. Int. J. Nanomed. 2019, 14, 8891–8903. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Snow, L.D.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. Springerplus 2016, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Bourkaib, M.C.; Gaudin, P.; Vibert, F.; Guiavarc’H, Y.; Delaunay, S.; Framboisier, X.; Humeau, C.; Chevalot, I.; Blin, J.-L. APTES modified SBA15 and meso-macro silica materials for the immobilization of aminoacylases from Streptomyces ambofaciens. Microporous Mesoporous Mater. 2021, 323, 111226. [Google Scholar] [CrossRef]
- Engelmann, C.; Ekambaram, N.; Johannsen, J.; Fellechner, O.; Waluga, T.; Fieg, G.; Liese, A.; Bubenheim, P. Enzyme Immobilization on Synthesized Nanoporous Silica Particles and their Application in a Bi-enzymatic Reaction. Chemcatchem 2020, 12, 2245–2252. [Google Scholar] [CrossRef]
- Yen, C.-C.; Chuang, Y.-C.; Ko, C.-Y.; Chen, L.-F.O.; Chen, S.-S.; Lin, C.-J.; Chou, Y.-L.; Shaw, J.-F. Immobilization of Chlamydomonas reinhardtii CLH1 on APTES-Coated Magnetic Iron Oxide Nanoparticles and Its Potential in the Production of Chlorophyll Derivatives. Molecules 2016, 21, 972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Razmjou, A.; Yek, S.M.-G.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. Nutr. 2020, 61, 3160–3196. [Google Scholar] [CrossRef] [PubMed]
- Du, P.D.; Hieu, N.T.; To, T.C.; Bach, L.G.; Tinh, M.X.; Mau, T.X.; Khieu, D.Q. Aminopropyl Functionalised MCM-41: Synthesis and Application for Adsorption of Pb(II) and Cd(II). Adv. Mater. Sci. Eng. 2019, 2019, 8573451. [Google Scholar] [CrossRef] [Green Version]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Kamiya, T.; Tanimoto, Y.; Fujii, N.; Negishi, T.; Suzuki, T.; Hatano, T.; Arimoto-Kobayashi, S. 2,6-Dimethoxy-1,4-benzoquinone, isolation and identification of anti-carcinogenic, anti-mutagenic and anti-inflammatory component from the juice of Vitis coignetiae. Food Chem. Toxicol. 2018, 122, 172–180. [Google Scholar] [CrossRef]
- Shin, K.-S. Oxidation of syringic acid by extracellular peroxidase of white-rot fungus, Pleurotus ostreatus. Mycoscience 1995, 36, 31–35. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Minard, R.D.; Bollag, J.-M. Oligomerization of Syringic Acid, a Lignin Derivative, by a Phenoloxidase. Soil Sci. Soc. Am. J. 1981, 45, 1100–1105. [Google Scholar] [CrossRef]









| Element (%) | 0 h | 2 h | 3 h | 4 h | 5 h |
|---|---|---|---|---|---|
| C | 38.70 | 3.80 | 1.18 | 0.40 | 0.04 |
| H | 6.00 | 0.44 | 0.49 | 0.29 | 0.38 |
| N | 0.45 | 0.37 | 0.11 | 0 | 0 |
| Scaffold | Immobilized Activity (U/g) | Efficiency (%) |
|---|---|---|
| Chitosan/Alginate (CA) | 0.7 | 0.9 |
| APTES/Chitosan/Alginate (ACA) | 1.9 | 1.9 |
| RHA/Chitosan/Alginate (RCA) | 2.6 | 3.1 |
| APTES/RHA/Chitosan/Alginate (ARCA) | 3.8 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuto, F.R.; Ciarlantini, C.; Chiappini, V.; Pietrelli, L.; Piozzi, A.; Girelli, A.M. Design of a 3D Amino-Functionalized Rice Husk Ash Nano-Silica/Chitosan/Alginate Composite as Support for Laccase Immobilization. Polymers 2023, 15, 3127. https://doi.org/10.3390/polym15143127
Scuto FR, Ciarlantini C, Chiappini V, Pietrelli L, Piozzi A, Girelli AM. Design of a 3D Amino-Functionalized Rice Husk Ash Nano-Silica/Chitosan/Alginate Composite as Support for Laccase Immobilization. Polymers. 2023; 15(14):3127. https://doi.org/10.3390/polym15143127
Chicago/Turabian StyleScuto, Francesca Romana, Clarissa Ciarlantini, Viviana Chiappini, Loris Pietrelli, Antonella Piozzi, and Anna M. Girelli. 2023. "Design of a 3D Amino-Functionalized Rice Husk Ash Nano-Silica/Chitosan/Alginate Composite as Support for Laccase Immobilization" Polymers 15, no. 14: 3127. https://doi.org/10.3390/polym15143127
APA StyleScuto, F. R., Ciarlantini, C., Chiappini, V., Pietrelli, L., Piozzi, A., & Girelli, A. M. (2023). Design of a 3D Amino-Functionalized Rice Husk Ash Nano-Silica/Chitosan/Alginate Composite as Support for Laccase Immobilization. Polymers, 15(14), 3127. https://doi.org/10.3390/polym15143127