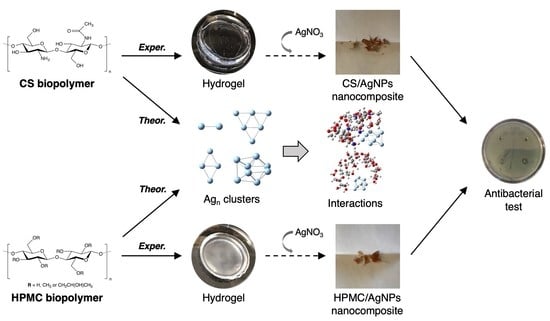
Biopolymer-Based Composite Hydrogels Embedding Small Silver Nanoparticles for Advanced Antimicrobial Applications: Experimental and Theoretical Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Polysaccharide-Based Hydrogels
2.3. Synthesis of Silver Nanocomposite Hydrogels
2.4. Characterization of Nanomaterials
2.5. Swelling–Deswelling Studies
2.6. Antibacterial Activity Assay
2.7. Computational Details
3. Results and Discussion
3.1. Preparation of CS and HPMC Hydrogels
3.2. Synthesis of CS/AgNPs and HPMC/AgNPs Composite Hydrogels
3.3. Characterization Results
3.4. Evaluation of Antimicrobial Activity
3.5. Theoretical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical Synthesis of Nanoparticles for Potential Antimicrobial Applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, E.A.; Shabalkin, I.D.; Krivoshapkin, P.V. Monometallic and alloy nanoparticles: A review of biomedical applications. J. Mater. Chem. B 2023, 11, 3054–3070. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, S.; Devi, S.; Pandian, K.; Devendiran, R.; Selvaraj, M. Sunlight-assisted synthesis of silver nanoparticles in zeolite matrix and study of its application on electrochemical detection of dopamine and uric acid in urine samples. Mater. Sci. Eng. C 2016, 69, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Mochrie, S.G.; Lurio, L.B.; Ruhm, A.; Lennox, R.B. Polymer-stabilized gold nanoparticles and their incorporation into polymer matrices. J. Am. Chem. Soc. 2001, 123, 10411–10412. [Google Scholar] [CrossRef]
- Sonawane, S.S.; Thakur, P.P.; Malika, M.; Ali, H.M. Recent Advances in the Applications of Green Synthesized Nanoparticle-based Nanofluids for the Environmental Remediation. Curr. Pharm. Biotechnol. 2023, 24, 164–187. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tawanda Tavengwa, N. Advances in the application of chitosan-based metal organic frameworks as adsorbents for environmental remediation. Carbohydr. Polym. 2022, 283, 119153. [Google Scholar] [CrossRef]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Valueva, S.V.; Borovikova, L.N.; Vylegzhanina, M.E.; Nazarova, O.V.; Panarin, E.F. Metal and Metalloid Nanoparticles Stabilized by (Bio)polymers: Spectral and Structural-Morphological Characteristics. Tech. Phys. 2022, 67, 258–266. [Google Scholar] [CrossRef]
- Silva, A.T.; Coelho, A.G.; Lopes, L.C.D.S.; Martins, M.V.; Crespilho, F.N.; Merkoçi, A.; Silva, W.C.D. Nano-assembled supramolecular films from chitosan-stabilized gold nanoparticles and cobalt (II) phthalocyanine. J. Braz. Chem. Soc. 2013, 24, 1237–1245. [Google Scholar] [CrossRef]
- Xia, C.; Jin, X.; Parandoust, A.; Sheibani, R.; Khorsandi, Z.; Montazeri, N.; Wu, Y.; Van Le, Q. Chitosan-supported metal nanocatalysts for the reduction of nitroaromatics. Int. J. Biol. Macromol. 2023, 239, 124135. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Parvez, M.A.K.; Balusamy, S.R.; Rahman, M.M.; Kim, J.H.; Akter, S. Chitosan-Coated Polymeric Silver and Gold Nanoparticles: Biosynthesis, Characterization and Potential Antibacterial Applications: A Review. Polymers 2022, 14, 5302. [Google Scholar] [CrossRef] [PubMed]
- Tokarek, K.; Hueso, J.L.; Kuśtrowski, P.; Stochel, G.; Kyzioł, A. Green Synthesis of Chitosan-Stabilized Copper Nanoparticles. Eur. J. Inorg. Chem. 2013, 2013, 4940–4947. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jung, J.; Kim, D.; Varaprasad, K.; Seo, J. Identification of silver cubic structures during ultrasonication of chitosan AgNO3 solution. Carbohydr. Polym. 2016, 152, 558–565. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.-N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Gao, C. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, X.; Lin, X.; Yi, W.; Jiang, J. An injectable metal nanoparticle containing cellulose derivative-based hydrogels: Evaluation of antibacterial and in vitro-vivo wound healing activity in children with burn injuries. Int. Wound J. 2022, 19, 666–678. [Google Scholar] [CrossRef]
- George, J.; Kumar, R.; Sajeevkumar, V.A.; Ramana, K.V.; Rajamanickam, R.; Abhishek, V.; Nadanasabapathy, S. Hybrid HPMC nanocomposites containing bacterial cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2014, 105, 285–292. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Riva, L.; Lotito, A.D.; Punta, C.; Sacchetti, A. Zinc and Copper-Loaded Nanosponges from Cellulose Nanofibers Hydrogels: New Heterogeneous Catalysts for the Synthesis of Aromatic Acetals. Gels 2022, 2022, 54. [Google Scholar] [CrossRef] [PubMed]
- Dzhardimalieva, G.I.; Uflyand, I.E. Preparation of metal-polymer nanocomposites by chemical reduction of metal ions: Functions of polymer matrices. J. Polym. Res. 2018, 25, 255. [Google Scholar] [CrossRef]
- Hasan, M.S.; Al Foisal, J.; Khan, G.M.A.; Jahan, R.; Hasanuzzaman, M.; Alam, M.S.; Karim, M.M.; Gafur, M.A.; Khan, M.A.; Sabur, M.A. Microfibrillated Cellulose-Silver Nanocomposite Based PVA Hydrogels and Their Enhanced Physical, Mechanical and Antibacterial Properties. J. Polym. Environ. 2022, 30, 2875–2887. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, J.; Jin, S.; Zheng, Y.; Gao, W.; Wu, D.; Yu, J.; Dai, Z. Cellulose-nanofibril-reinforced hydrogels with pH sensitivity and mechanical stability for wound healing. Mater. Lett. 2022, 323, 132596. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.S.; Hu, X.H.; Chu, L.Q. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial Lignin-Based Polyurethane/Ag Composite Foams for Improving Wound Healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef]
- Megarajan, S.; Ameen, F.; Singaravelu, D.; Islam, M.A.; Veerappan, A. Synthesis of N-myristoyltaurine stabilized gold and silver nanoparticles: Assessment of their catalytic activity, antimicrobial effectiveness and toxicity in zebrafish. Environ. Res. 2022, 212, 113159. [Google Scholar] [CrossRef]
- Naghmachi, M.; Raissi, A.; Baziyar, P.; Homayoonfar, F.; Amirmahani, F.; Danaei, M. Green synthesis of silver nanoparticles (AgNPs) by Pistacia terebinthus extract: Comprehensive evaluation of antimicrobial, antioxidant and anticancer effects. Biochem. Biophys. Res. Commun. 2022, 608, 163–169. [Google Scholar] [CrossRef] [PubMed]
- De Moura, M.R.; Mattoso, L.H.; Zucolotto, V. Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C.; Broker, H.; Merritt, E.; Campbell, J.; Cunningham, R.; Denholm, D.; Elber, G.; Fearick, R.; Grammes, C. Gnuplot 5.2: An Interactive Plotting Program, Official Gnuplot Documentation. 2019. Available online: http://sourceforge.net/projects/gnuplot (accessed on 24 January 2023).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Premkumar, T.; Lee, K.; Geckeler, K.E. Fabrication of silver nanoparticles in hydrogel networks. Macromol. Rapid Commun. 2006, 27, 1346–1354. [Google Scholar] [CrossRef]
- Murthy, P.K.; Mohan, Y.M.; Varaprasad, K.; Sreedhar, B.; Raju, K.M. First successful design of semi-IPN hydrogel–silver nanocomposites: A facile approach for antibacterial application. J. Colloid Interface Sci. 2008, 318, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Varaprasad, K.; Raghavendra, G.M.; Sadiku, E.R.; Mohana Raju, K.; Amalraj, J. Green synthesis of tea Ag nanocomposite hydrogels via mint leaf extraction for effective antibacterial activity. J. Biomater. Sci. Polym. Ed. 2017, 28, 1588–1602. [Google Scholar] [CrossRef]
- Bonačić-Koutecký, V.; Češpiva, L.; Fantucci, P.; Koutecký, J. Effective core potential-configuration interaction study of electronic structure and geometry of small neutral and cationic Ag n clusters: Predictions and interpretation of measured properties. J. Chem. Phys. 1993, 98, 7981–7994. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree–Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Sun, G.; Liang, T.; Tan, W.; Wang, L. Rheological behaviors and physical properties of plasticized hydrogel films developed from κ-carrageenan incorporating hydroxypropyl methylcellulose. Food Hydrocoll. 2018, 85, 61–68. [Google Scholar] [CrossRef]
- Menter, P. Acrylamide Polymerization—A Practical Approach. Bio-Rad Tech. Note 2000, 1156, 24. [Google Scholar]
- Wang, J.; Ugaz, V.M. Using in situ rheology to characterize the microstructure in photopolymerized polyacrylamide gels for DNA electrophoresis. Electrophoresis 2006, 27, 3349–3358. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Babu, V.R.; Kim, C.; Kim, S.; Ahn, C.; Lee, Y.I. Development of semi-interpenetrating carbohydrate polymeric hydrogels embedded in silver nanoparticles and its facile studies on E. coli. Carbohydr. Polym. 2010, 81, 196–202. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef] [Green Version]
- Mohan, Y.M.; Vimala, K.; Thomas, V.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.K.; Raju, K.M. Controlling of silver nanoparticles structure by hydrogel networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Der Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Peng, H.; Yang, A.; Xiong, J. Green, microwave-assisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium. Carbohydr. Polym. 2013, 91, 348–355. [Google Scholar] [CrossRef]
- Kumar, P.; Choonara, Y.; Toit, L.; Modi, G.; Naidoo, D.; Pillay, V. Novel high-viscosity polyacrylamidated chitosan for neural tissue engineering: Fabrication of anisotropic neurodurable scaffold via molecular disposition of persulfate-mediated polymer slicing and complexation. Int. J. Mol. Sci. 2012, 13, 13966–13984. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, G.R.; Ettehadi, S.; Amini, M.; Sabzi, M. Synthesis and characterization of hydroxypropyl methylcellulose-g-poly (acrylamide)/LAPONITE® RD nanocomposites as novel magnetic-and pH-sensitive carriers for controlled drug release. RSC Adv. 2015, 5, 44516–44523. [Google Scholar] [CrossRef]
- Ravindra, S.; Mohan, Y.M.; Reddy, N.N.; Raju, K.M. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 31–40. [Google Scholar] [CrossRef]
- Haldar, U.; Nandi, M.; Maiti, B.; De, P. POSS-induced enhancement of mechanical strength in RAFT-made thermoresponsive hydrogels. Polym. Chem. 2015, 6, 5077–5085. [Google Scholar] [CrossRef]
- Mandal, S.K.; Brahmachari, S.; Das, P.K. In Situ Synthesised Silver Nanoparticle-Infused l-Lysine-Based Injectable Hydrogel: Development of a Biocompatible, Antibacterial, Soft Nanocomposite. ChemPlusChem 2014, 79, 1733–1746. [Google Scholar]
- Alarcon, E.I.; Udekwu, K.I.; Noel, C.W.; Gagnon, L.B.P.; Taylor, P.K.; Vulesevic, B.; Simpson, M.J.; Gkotzis, S.; Islam, M.M.; Lee, C.J.; et al. Safety and efficacy of composite collagen–silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale 2015, 7, 18789–18798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakia, M.; Koo, J.M.; Kim, D.; Ji, K.; Huh, P.; Yoon, J.; Yoo, S.I. Development of silver nanoparticle-based hydrogel composites for antimicrobial activity. Green Chem. Lett. Rev. 2020, 13, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Saruchi; Medha; Thakur, S.; Kaith, B.S.; Sharma, N.; Ansar, S.; Pandey, S.; Kuma, V. Biopolymer starch-gelatin embedded with silver nanoparticle–based hydrogel composites for antibacterial application. Biomass Convers. Biorefinery 2022, 12, 5363–5384. [Google Scholar] [CrossRef]
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lampanee, S.; Kulchat, S. Enhanced wound healing properties of guar gum/curcumin-stabilized silver nanoparticle hydrogels. Sci. Rep. 2021, 11, 21836. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sánchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2021, 254, 117302. [Google Scholar] [CrossRef]
- Pollini, M.; Russo, M.; Licciulli, A.; Sannino, A.; Maffezzoli, A. Characterization of antibacterial silver coated yarns. J. Mater. Sci. Mater. Med. 2009, 20, 2361. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Lakshmanan, A.; Ping, A.T.; Chin, J.M.; Hauser, C.A. In situ synthesis of size-controlled, stable silver nanoparticles within ultrashort peptide hydrogels and their anti-bacterial properties. Biomaterials 2014, 35, 7535–7542. [Google Scholar] [CrossRef]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Angeles Corcuera, M.; Gabilondo, N. Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2020, 143, 825–832. [Google Scholar] [CrossRef]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cao, X.; et al. Antibacterial nanomaterials: Mechanisms, impacts on antimicrobial resistance and design principles. Angew. Chem. Int. Ed. 2023, 62, e202217345. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; Amalraj, J. Chitosan capped copper oxide/copper nanoparticles encapsulated microbial resistant nanocomposite films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malola, S.; Häkkinen, H. Prospects and challenges for computer simulations of monolayer-protected metal clusters. Nat. Commun. 2021, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Camarada, M.B. DFT investigation of the interaction of gold nanoclusters with poly (amidoamine) PAMAM G0 dendrimer. Chem. Phys. Lett. 2016, 654, 29–36. [Google Scholar] [CrossRef]
- Pestov, A.; Nazirov, A.; Modin, E.; Mironenko, A.; Bratskaya, S. Mechanism of Au (III) reduction by chitosan: Comprehensive study with 13C and 1H NMR analysis of chitosan degradation products. Carbohydr. Polym. 2015, 117, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Utilization of hydroxypropyl cellulose for green and efficient synthesis of silver nanoparticles. Carbohydr. Polym. 2011, 86, 1615–1622. [Google Scholar] [CrossRef]
- Saldías, C.; Leiva, A.; Quezada, C.; Jaque, P.; Gargallo, L.; Radic, D. Structural effects of amphiphilic block copolymers on the gold nanoplates synthesis. Experimental and theoretical study. Eur. Polym. J. 2011, 47, 1866–1876. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Applications to organic chemistry. J. Org. Chem. 1989, 54, 1423–1430. [Google Scholar] [CrossRef]
- Ezzat, H.; Menazea, A.A.; Omara, W.; Basyouni, O.H.; Helmy, S.A.; Mohamed, A.A.; Tawfik, W.; Ibrahim, M. DFT: B3LYP/LANL2DZ study for the removal of Fe, Ni, Cu, As, Cd and Pb with Chitosan. Biointerface Res. Appl. Chem. 2020, 10, 7002–7010. [Google Scholar]
- Bursch, M.; Mewes, J.M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef] [PubMed]











| Hydrogel | Polymer | AAm (g) | MBA (mM) | TMEDA (mM) | APS (mM) | Sg/g |
|---|---|---|---|---|---|---|
| H-0 (control) | – | 1.0 | 6.48 | 8.62 | 21.91 | 13.2 |
| CS-2 | 2.0 mg/ml | 1.0 | 6.48 | 8.62 | 21.91 | 18.9 |
| CS-6.92 | 6.92 mg/ml | 1.0 | 6.48 | 8.62 | 21.91 | 23.2 |
| HPMC-0.5 | 0.5% (w/v) | 1.0 | 6.48 | 8.62 | 21.91 | 21.5 |
| HPMC-3 | 3.0% (w/v) | 1.0 | 6.48 | 8.62 | 21.91 | 22.7 |
| Medium, Matrices for NPs | Shape | Reported Size of NPs (nm) | Reference |
|---|---|---|---|
| Aq. suspension, PVP | Spherical | 20–80 | [5] |
| Aq. suspension, CS | Cubic | – | [14] |
| Aq. suspension, HPMC | Spherical | 3–17 | [16] |
| Aq. suspension | Cubic | 26 | [21] |
| Aq. suspension | Spherical | 2–3 | [24] |
| Film, HPMC | – | 41–100 | [27] |
| Hydrogel | Cubic | ~1 up to 80 | [48] |
| Aq. suspension | Spherical | 8.3–14.8 | [50] |
| Hydrogel | Spherical | 35–40 | [55] |
| Hydrogel, collagen | Spherical | 3.5 ± 0.04 | [56] |
| Hydrogel, curcumin | – | 18.24 ± 4.20 | [57] |
| Hydrogel | Spherical | ~12 | [58] |
| Hydrogel, starch | Semi-spherical | 4–58 | [59] |
| System | Total Energy | ∆Ecomplexation | ∆q (Agn) | H-L Gap | IP | EA |
|---|---|---|---|---|---|---|
| CS | −3184.405 | – | – | 6.378 | 6.411 | 0.034 |
| Ag2–CS | −3476.020 | −25.634 | −0.199 | 3.319 | 5.355 | 2.037 |
| Ag4–CS | −3767.628 | −30.421 | −0.323 | 2.311 | 4.515 | 2.204 |
| Ag6–CS | −4059.253 | −19.857 | −0.410 | 2.747 | 5.023 | 2.277 |
| Ag8–CS | −4350.901 | −37.288 | −0.483 | 2.465 | 4.598 | 2.134 |
| HPMC | −3363.556 | – | – | 6.767 | 6.788 | 0.021 |
| Ag2–HPMC | −3655.173 | −26.326 | −0.134 | 3.451 | 5.133 | 1.683 |
| Ag4–HPMC | −3946.775 | −27.435 | −0.300 | 2.327 | 4.390 | 2.063 |
| Ag6–HPMC | −4238.411 | −23.931 | −0.317 | 2.760 | 5.115 | 2.355 |
| Ag8–HPMC | −4530.029 | −22.385 | −0.359 | 2.546 | 4.540 | 1.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, M.A.; Amalraj, J.; Santos, L.S. Biopolymer-Based Composite Hydrogels Embedding Small Silver Nanoparticles for Advanced Antimicrobial Applications: Experimental and Theoretical Insights. Polymers 2023, 15, 3370. https://doi.org/10.3390/polym15163370
Rojas MA, Amalraj J, Santos LS. Biopolymer-Based Composite Hydrogels Embedding Small Silver Nanoparticles for Advanced Antimicrobial Applications: Experimental and Theoretical Insights. Polymers. 2023; 15(16):3370. https://doi.org/10.3390/polym15163370
Chicago/Turabian StyleRojas, Moises A., John Amalraj, and Leonardo S. Santos. 2023. "Biopolymer-Based Composite Hydrogels Embedding Small Silver Nanoparticles for Advanced Antimicrobial Applications: Experimental and Theoretical Insights" Polymers 15, no. 16: 3370. https://doi.org/10.3390/polym15163370
APA StyleRojas, M. A., Amalraj, J., & Santos, L. S. (2023). Biopolymer-Based Composite Hydrogels Embedding Small Silver Nanoparticles for Advanced Antimicrobial Applications: Experimental and Theoretical Insights. Polymers, 15(16), 3370. https://doi.org/10.3390/polym15163370