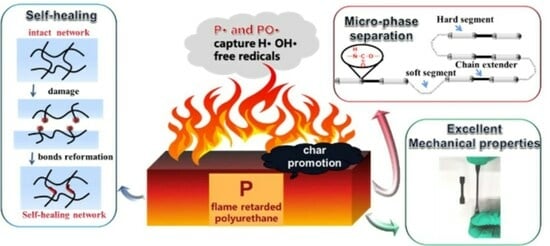
Strategy for Constructing Phosphorus-Based Flame-Retarded Polyurethane Elastomers for Advanced Performance in Long-Term
Abstract
:1. Introduction to Polyurethane Elastomers

2. Research the Status of Reactive Flame-Retarded Polyurethane Elastomer
2.1. From Halogen-Containing Flame Retardant to Halogen-Free Flame Retardant
2.2. P-Containing Reactive Flame Retardant
2.3. N-Containing Reactive Flame Retardant
2.4. Silicon-Containing Reactive Flame Retardant
2.5. Boron-Containing Reactive Flame Retardant
2.6. Selenium-Containing Reactive Flame Retardant
2.7. Thermal Cross-Linking Reactive Flame Retardant
3. Flame Retarding Mechanism of P-FRs in PUE
3.1. Thermal Decomposition Behavior of P-FR Polyurethane
3.2. General Flame Retarding Mechanisms of P-FRs
3.3. Effect of Phosphorus Oxidation State on Flame Retarding Mechanism
4. Inherent Phosphorus-Containing Flame Retardants Applied in PUE
4.1. Application of Phosphorus-Containing Chain Extender in Flame-Retarded PUE
4.2. Application of Phosphorus-Containing Polyols in Flame-Retarded PUE
5. Phosphorus-Heteroatom Synergistic Flame Retardants
5.1. P-N Synergistic Flame Retardants
5.1.1. P-N Synergistic Chain Extenders
5.1.2. P-N synergistic Reactive Polyols
5.1.3. P-N Synergistic DOPO-Based Flame-Retarded Polyurethane
5.2. Other P-Heteroatom Synergistic Flame Retardants
6. Perspective on Future Development of P-FRs-Based TPU
6.1. Design of P-FRs Inspired from the Intrinsic Flame Retarded Polyurethane Foam
6.2. Design of P-FRs Inspired from the Intrinsic Flame Retarded Epoxy Resin
6.3. More Environmentally Friendly Flame Retarded Polyurethane with Biological Based Material
6.4. Functional Flame-Retarded Polyurethane
6.4.1. Application of Flame-Retarded Polyurethane in Polyelectrolyte
6.4.2. Self-Healing Flame-Retarded Polyurethane
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Chemical Structure and Abbreviation
| Chemical structure | Abbreviation | Full name |
 | Phosphonate diol | Phosphonate diol |
 | PAMAD | Phytic acid- 3-(benzyloxy)-2- ((benzyloxy)methyl)-N-(4,6-diamino-1,3,5-triazin-2-yl)- 2-methyl propanamide |
 | 1,2-P-POSS | 1,2-propanediol isobutyl POSS |
 | Diglyceride borate | Diglyceride borate |
 | DiSe | Di(1-hydroxyethylene) Diselenide |
 | PEPE | 4-(phenylethynyl) di(ethylene glycol) phthalate |
 | NPG | Neopentyl glycol |
 | EPPD | Synthesis of 2-ethyl-2-(2-oxo-5,5-dimethyl-1,3,2- dioxaphosphorinanyl-2-methylene)-1,3-propanediol |
 | HMCPP | (3-(2,2-bis(hydroxymethyl)butoxy)-3-oxopropyl)(phenyl)phosphinic acid |
 | BPAMPP | bis(4-(2-(4-hydroxyphenyl)propane-2-yl)phenyl) phenyl phosphonate |
 | THPO | tris(hydroxymethyl)phosphine oxide |
 | PPO | (1,3-bis(2-hydroxypropoxy)propane-2-yl)phosphonic acid |
 | OP | Phosphorous polyol |
 | PCEPEP-PO | 2-hydroxyethyl 3-((2-hydroxyethoxy)(phenyl)phosphoryl)propanoate |
 | ||
| FRPE | flame-retardant polyester diol | |
 | PDNP | pentaerythritol di-N-hydroxyethyl phosphamide |
 | ODDP | octahydro-2,7- di(N,N-dimethylamino)-1,6,3,8,2,7-di-oxadiazadiphosphecine |
 | PNMPD | 2-(5,5-dimethyl-2-oxo-2l5 -1,3,2- dioxaphosphinan-2-ylamino)-2-methyl-propane-1,3-diol |
 | BH | [bis(2-hydroxyethyl)amino]-methyl- phosphonic acid dimethyl ester |
 | PSK2 | 4-((E)-(hydroxyimino)methyl)phenyl bis(4-((Z)-(hydroxyimino)methyl)phenyl) phosphate |
 | TFP | tri(2-furyl) phosphoramide |
 | TMCTP | Tri-Maleimide End-Capped Cyclotriphosphazene |
 | BAPPO | bis(4-amino phenoxy)phenyl phosphine oxide |
 | BSPB | Benzoguanamine spirocyclic pentaerythritol bisphosphonate |
 | OH2-N2P4 | (bis((bis((l3-oxidaneylidyne)methyl)phosphoryl)methyl)amino)methyl 4-(2-(3-((bis((bis((l3-oxidaneylidyne)methyl)phosphoryl)methyl)glycyl)oxy)-2-hydroxypropoxy)ethoxy)-3-hydroxybutyrate |
 | JZP | 2-(((diethoxyphosphoryl)methyl)(2-hydroxyethyl)amino)ethyl (6-hydroxyphenyl) adipate |
 | PNP | 18-(bis(hydroxymethyl)amino)-10-hydroxy-18-oxooctadecan-9-yl dipentyl phosphate |
 | FRD | 10-(1,4-dicarboxyl)-9,10-dihydro-9-oxa-10-phosphaphenanthrene- 10-oxide-diethyl bis(2-hydroxyethyl) aminomethylphosphonate |
 | DOPO-DAM | 9,10-dihydro-9-oxa-10- [N, N-bis-(2- hydroxyethylamino-methyl)]-10-phosphaphenanthrene-10- oxide |
 | PHID | 4-DOPO-((3-hydroxypropyl) imino) methyl) phenol |
 | DOPD-HAMB | 4-DOPO-(((4-hydroxyphenyl)amino)methyl)benzene- 1,3-diol |
 | P-S | phosphonate diol II |
 | P-Si(PHID+Si-OH) | 4-DOPO-((3-hydroxypropyl) imino) methyl) phenol Hydroxy silicone oil |
 | P-N-S | vanillin-based polyol with flame-retardant group |
 | HAMPP | 2-((Bis(2-hydroxyethyl) amino) methyl)-5,5-dimethyl-1,3,2-dioxaphosphinane 2-oxide |
 | PPA-PO-polyol | 2-hydroxypropyl isobutyl phenylphosphonate |
 | PDEO | ethane-1,2-diyl bis(2-hydroxyethyl) bis(phenylphosphonate) |
 | DMOP | bis(2-((2-hydroxyethyl)amino)ethyl) methylphosphonate |
 | aminophosphonate polyether polyol | aminophosphonate polyether polyol |
| - | PEG | Polyethylene Glycol |
| - | PPG | Polypropylene Glycol |
| - | PTMEG | Polytetramethylene ether Glycol |
| - | TDI | Toluene di-isocyanate |
| - | MDI | Methylenediphenyl di-isocyanate |
| - | HDI | Hexamethylene di-isocyanate |
| - | IPDI | Isophorone di-isocyanate |
| - | HMDI | 4,4′-diisocyanate dicyclohexylmethane |
References
- Aitken, R.R. Thermoplastic polyurethane elastomers based on aliphatic diisocyanates: Thermal transitions. Polymer 1976, 18, 197–198. [Google Scholar] [CrossRef]
- Pztrov, Z.S. Polyurethane elastomers. Prog. Polym. Sci. 1991, 16, 695–836. [Google Scholar]
- Hong, J.-L. Degree of phase separation in polyether-polyurethane copolymers with different chemical structures of hard segments. Polymer 1992, 33, 4347–4351. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.-Y.; Huang, S.-C.; Wei, W.-C.; Yang, A.-H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Naureen, B.; Haseeb, A.; Basirun, W.J.; Muhamad, F. Recent advances in tissue engineering scaffolds based on polyurethane and modified polyurethane. Mater. Sci. Eng. C-Mater. 2021, 118, 111228. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Cong, K.; Liu, Z.; He, J.; Yang, R. Preparation and performance of polyether elastomer with a combination of polyurethane and polytriazole. J. Appl. Polym. Sci. 2021, 139, 51842. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Ginzburg, V.V.; Schiller, K.S.; Wendt, B.L. Design, polymerization, and properties of high performance thermoplastic polyurethane elastomers from seed-oil derived soft segments. Polymer 2013, 54, 1350–1360. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Amiralian, N.; Annamalai, P.K.; Edwards, G.; Chaleat, C.; Martin, D.J. Scalable processing of thermoplastic polyurethane nanocomposites toughened with nanocellulose. Chem. Eng. J. 2016, 302, 406–416. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile materials and sustainable problem solvers for today's challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Toldy, A.; Harakály, G.; Szolnoki, B.; Zimonyi, E.; Marosi, G. Flame retardancy of thermoplastics polyurethanes. Polym. Degrad. Stab. 2012, 97, 2524–2530. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Yuan, B.; Hu, Y.; Song, L. Functionalized graphene oxide for fire safety applications of polymers: A combination of condensed phase flame retardant strategies. J. Mater. Chem. A 2012, 22, 23057–23063. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Wang, Y.-Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Lu, S.; Feng, Y.; Zhang, P.; Hong, W.; Chen, Y.; Fan, H.; Yu, D.; Chen, X. Preparation of Flame-Retardant Polyurethane and Its Applications in the Leather Industry. Polymers 2021, 13, 1730. [Google Scholar] [CrossRef]
- Nazir, R.; Gaan, S. Recent developments in P(O/S)–N containing flame retardants. J. Appl. Polym. Sci. 2019, 137, 218–244. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting-How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Qi, X.; Qian, L. The pyrolysis behaviors of phosphorus-containing organosilicon compound modified APP with different polyether segments and their flame retardant mechanism in polyurethane foam. Compos. Part B-Eng. 2019, 173, 106784. [Google Scholar] [CrossRef]
- Du, H.; Ren, J.; Fu, X.; Zhang, W.; Yang, R. Simultaneous improvements of the fire safety, mechanical properties and water resistance of vinyl ester resin composites by introducing microencapsulated ammonium polyphosphate by polytriazole. Compos. Part B-Eng. 2022, 238, 109908. [Google Scholar] [CrossRef]
- Li, D.; Liu, L.; Zhang, Z.; Xu, M.; Xu, Y.; Qian, L. An urethane-based phosphonate ester for improving flame retardancy and smoke suppression of thermoplastic polyurethane. Polym. Degrad. Stab. 2021, 188, 109568. [Google Scholar] [CrossRef]
- Cai, W.; Wang, B.; Liu, L.; Zhou, X.; Chu, F.; Zhan, J.; Hu, Y.; Kan, Y.; Wang, X. An operable platform towards functionalization of chemically inert boron nitride nanosheets for flame retardancy and toxic gas suppression of thermoplastic polyurethane. Compos. Part B-Eng. 2019, 178, 107462. [Google Scholar] [CrossRef]
- Camino, G.C., L.; Di Cortemiglia, M.L. Overview of Fire Retardant Mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Schartel, B. It Takes Two to Tango: Synergistic Expandable Graphite–Phosphorus Flame Retardant Combinations in Polyurethane Foams. Polymers 2022, 14, 2562. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Rodríguez, J.F.; Velencoso, M.M.; Serrano, Á.; Ramos, M.J. DMSO as solvent on the synthesis of flame-retardant polyether polyols. J. Appl. Polym. Sci. 2019, 136, 47042. [Google Scholar] [CrossRef]
- Chen, H.; Deng, C.; Zhao, Z.-Y.; Wan, L.; Yang, A.-H.; Wang, Y.-Z. Novel piperazine-containing oligomer as flame retardant and crystallization induction additive for thermoplastics polyurethane. Chem. Eng. J. 2020, 400, 125941. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Zhang, Q.; Lu, G.-P.; Lin, Y. Tannic acid as cross-linker and flame retardant for preparation of flame-retardant polyurethane elastomers. React. Funct. Polym. 2022, 181, 105454. [Google Scholar] [CrossRef]
- Borse, P.; Naiker, V.; Mestry, S.; Shah, V.; Mhaske, S.T. Development of phosphorous-based melamine–vanillin imine precursor for flame-retardant polyurethane coating. Polym. Bull. 2022, 80, 10473–10492. [Google Scholar] [CrossRef]
- Yin, X.; Li, L.; Pang, H.; Luo, Y.; Zhang, B. Halogen-free instinct flame-retardant waterborne polyurethanes: Composition, performance, and application. RSC Adv. 2022, 12, 14509–14520. [Google Scholar] [CrossRef] [PubMed]
- Spirckel, M.; Regnier, N.; Mortaignea, B.; Youssef, B.; Bunel, C. Thermal degradation and fire performance of new phosphonate polyurethanes. Polym. Degrad. Stab. 2002, 78, 211–218. [Google Scholar] [CrossRef]
- Gu, L.; Shi, Y.; Zhang, L. Synthesis and characterization of bio-based “three sources in one” intumescent flame retardant monomer and the intrinsic flame retardant waterborne polyurethane. J. Polym. Res. 2022, 29, 189. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, C.K.; Kwon, Y. Ablation and fire-retardant properties of hydroxyl-terminated polybutadiene-based polyurethane-g-polyhedral oligomeric silsesquioxane composites. High Perform Polym. 2014, 27, 749–757. [Google Scholar] [CrossRef]
- Xia, L.; Liu, J.; Li, Z.; Wang, X.; Wang, P.; Wang, D.; Hu, X. Synthesis and flame retardant properties of new boron-containing polyurethane. J. Macramol. Sci. A 2020, 57, 560–568. [Google Scholar] [CrossRef]
- Xie, M.; Jia, D.; Hu, J.; He, J.; Li, X.; Yang, R. Fabrication of Enhanced Mechanical Properties and Intrinsic Flame-Retardant Polyurethane Elastomer Containing 4-(Phenylethynyl) Di(Ethylene Glycol) Phthalate. Polymers 2021, 13, 2388. [Google Scholar] [CrossRef]
- Shang, X.; Jin, Y.; Du, W.; Bai, L.; Zhou, R.; Zeng, W.; Lin, K. Flame-Retardant and Self-Healing Waterborne Polyurethane Based on Organic Selenium. ACS Appl. Mater. Int. 2023, 15, 16118–16131. [Google Scholar] [CrossRef]
- Chu, F.; Qiu, S.; Zhou, Y.; Zhou, X.; Cai, W.; Zhu, Y.; He, L.; Song, L.; Hu, W. Novel glycerol-based polymerized flame retardants with combined phosphorus structures for preparation of high performance unsaturated polyester resin composites. Compos. Part B-Eng. 2022, 233, 109647. [Google Scholar] [CrossRef]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials 2017, 10, 784. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, R.; He, J. Investigations of Thermoplastic Poly(imide-urethanes) Flame-Retarded by Hydroxyl-Terminated Poly(dimethylsiloxane). Ind. Eng. Chem. Res. 2014, 53, 9714–9720. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Camino, G. Metal functionalized POSS as fire retardants in polypropylene. Polym. Degrad. Stab. 2006, 91, 2275–2281. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- He, Q.; Song, L.; Hu, Y.; Zhou, S. Synergistic effects of polyhedral oligomeric silsesquioxane (POSS) and oligomeric bisphenyl A bis(diphenyl phosphate) (BDP) on thermal and flame retardant properties of polycarbonate. J. Mater. Sci. 2009, 44, 1308–1316. [Google Scholar] [CrossRef]
- Ye, X.M.; Li, J.J.; Zhang, W.C.; Yang, R.J.; Li, J.R. Fabrication of eco-friendly and multifunctional sodium-containing polyhedral oligomeric silsesquioxane and its flame retardancy on epoxy resin. Compos. Part B-Eng. 2020, 191, 107961. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, X.; Jiang, Y.; Qiao, L.; Zhang, W.; Pan, Y.-T.; Yang, R.; Li, J.; Li, Y. Controllable dimensions and regular geometric architectures from self-assembly of lithium-containing polyhedral oligomeric silsesquioxane: Build for enhancing the fire safety of epoxy resin. Compos. Part B-Eng. 2022, 229, 109483. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, H.-B.; Ni, Y.-P.; Fu, T.; Wu, W.-S.; Wang, X.-L.; Wang, Y.-Z. 3D printable robust shape memory PET copolyesters with fire safety via π-stacking and synergistic crosslinking. J. Mater. Chem. A. 2019, 7, 17037–17045. [Google Scholar] [CrossRef]
- Huang, S.-C.; Deng, C.; Zhao, Z.-Y.; Chen, H.; Gao, Y.-Y.; Wang, Y.-Z. Phosphorus-containing organic-inorganic hybrid nanoparticles for the smoke suppression and flame retardancy of thermoplastic polyurethane. Polym. Degrad. Stab. 2020, 178, 109179. [Google Scholar] [CrossRef]
- Yang, A.-H.; Deng, C.; Chen, H.; Wei, Y.-X.; Wang, Y.-Z. A novel Schiff-base polyphosphate ester: Highly-efficient flame retardant for polyurethane elastomer. Polym. Degrad. Stab. 2017, 144, 70–82. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Chen, L.; Yang, J.-C.; Ge, X.-G.; Wang, Y.-Z. A novel flame-retardant-free copolyester: Cross-linking towards self extinguishing and non-dripping. J. Mater. Chem. A 2012, 22, 19849–19857. [Google Scholar] [CrossRef]
- Chen, H.; Deng, C.; Zhao, Z.-Y.; Huang, S.-C.; Wei, Y.-X.; Wang, Y.-Z. Novel alkynyl-containing phosphonate ester oligomer with high charring capability as flame retardant additive for thermoplastic polyurethane. Compos. Part B-Eng. 2020, 199, 108315. [Google Scholar] [CrossRef]
- Pandya, M.V. Thermal Behavior of Cast Polyurethane Elastomers. J. Appl. Polym. Sci. 1988, 35, 1803–1815. [Google Scholar] [CrossRef]
- Zhang, J. Synthesis and properties of waterborne polyurethane modified by bisphenol A type phosphorus-containing diol. Fine Chem. 2020, 37, 1–7. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Li, Q.Y.; Liang, X.T. Synthesis and characterization of flame-retardant polyurethane based on new chain extenders. Polym. Int. 2022, 71, 1193–1200. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of phosphorus valency on thermal behaviour of flame retarded polyurethane foams. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame Retardancy Mechanisms of Aluminium Phosphinate in Combination with Melamine Cyanurate in Glass-Fibre-Reinforced Poly(1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Braun, U. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, S.; Fan, H.; Chen, Y.; Yan, J. Flame retardancy and hydrolysis resistance of waterborne polyurethane bearing organophosphate moieties lateral chain. Prog. Org. Coat. 2015, 89, 170–180. [Google Scholar] [CrossRef]
- Chiu, S.-H.; Wu, C.-L.; Lee, H.-T.; Gu, J.-H.; Suen, M.-C. Synthesis and characterisation of novel flame retardant polyurethanes containing designed phosphorus units. J. Polym. Res. 2016, 23, 205. [Google Scholar] [CrossRef]
- Çetinkaya, I.C.; Yüksel, G.; Macit, C.; Üreyen, M.E.; Eren, T. Synthesis and Characterization of Polyphosphonates and Polyurethanes Using Chalcone and DOPO-Chalcone as a Flame Retardant. ACS Appl. Polym. Mater. 2021, 3, 5277–5290. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Luo, Y. Synthesis and Characterization of Multifunctional Two-Component Waterborne Polyurethane Coatings: Fluorescence, Thermostability and Flame Retardancy. Polymers 2017, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Velencoso, M.M.; Ramos, M.J.; Klein, R.; De Lucas, A.; Rodriguez, J.F. Thermal degradation and fire behaviour of novel polyurethanes based on phosphate polyols. Polym. Degrad. Stab. 2014, 101, 40–51. [Google Scholar] [CrossRef]
- Zagozdzon, I.; Parcheta, P.; Datta, J. Novel Cast Polyurethanes Obtained by Using Reactive Phosphorus-Containing Polyol: Synthesis, Thermal Analysis and Combustion Behaviors. Materials 2021, 14, 2699. [Google Scholar] [CrossRef]
- Cai, C.; Sun, Q.; Zhang, K.; Bai, X.; Liu, P.; Li, A.; Lyu, Z.; Li, Q. Flame-retardant thermoplastic polyurethane based on reactive phosphonate polyol. Fire Mater. 2021, 46, 130–137. [Google Scholar] [CrossRef]
- Wang, H. Synthesis of reactive DOPO-based flame retardant and its application in polyurethane elastomers. Polym. Degrad. Stab. 2021, 183, 109440. [Google Scholar] [CrossRef]
- Patel, R.H.; Shah, M.D.; Patel, H.B. Synthesis and Characterization of Structurally Modified Polyurethanes Based on Castor Oil and Phosphorus-Containing Polyol for Flame-Retardant Coatings. Int. J. Polym. Anal. Charact. 2011, 16, 107–117. [Google Scholar] [CrossRef]
- Sun, C.; Bu, X.; Yang, T.; Qiao, C.; Ji, X.; Tao, F.; Gai, L.; Liu, L. Degradable Waterborne Polyurethane with Flame Retardancy and High Mechanical Strength via Synergy of Hydrogen Bonds. ACS Appl. Polym. Mater. 2023, 5, 5360–5369. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.T.; Guo, Z.H.; Fang, Z.P.; Chen, P.; Li, J. Effect of a bio-based copolymer containing lysine, dopamine and triazine on flame retardancy and mechanical properties of thermoplastic polyurethane/ammonium polyphosphate. Eur. Polym. J. 2023, 188, 111938. [Google Scholar] [CrossRef]
- Hu, W.-J.; Li, Y.-M.; Pang, Y.-Y.; Li, Y.-R.; Wang, D.-Y. The preparation of phosphorus and nitrogen-containing structure towards the enhancement of flame retardancy for thermoplastic polyurethane elastomer. Colloid Surface A. 2023, 656, 130375. [Google Scholar] [CrossRef]
- Kesavarao Sykam, S.S. Pratyay Basak. 1,2,3-Triazole mediated, non-halogenated phosphorus containing protective coatings from castor oil: Flame retardant and anti-corrosion applications. Prog. Org. Coat. 2023, 178, 107475. [Google Scholar] [CrossRef]
- Wang, S.; Du, Z.; Cheng, X.; Liu, Y.; Wang, H. Synthesis of a phosphorus- and nitrogen-containing flame retardant and evaluation of its application in waterborne polyurethane. J. Appl. Polym. Sci. 2018, 135, 46093. [Google Scholar] [CrossRef]
- Gu, L.; Luo, Y. Flame Retardancy and Thermal Decomposition of Phosphorus-Containing Waterborne Polyurethanes Modified by Halogen-Free Flame Retardants. Ind. Eng. Chem. Res. 2015, 54, 2431–2438. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Fan, H.; Tian, S.; Chen, Y.; Yan, J. Waterborne polyurethane conjugated with novel diol chain-extender bearing cyclic phosphoramidate lateral group: Synthesis, flammability and thermal degradation mechanism. RSC Adv. 2016, 6, 56610–56622. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Fu, X.; Du, Z.; Wang, H.; Cheng, X. Highly effective flame-retarded polyester diol with synergistic effects for waterborne polyurethane application. J. Appl. Polym. Sci. 2019, 137, 48444. [Google Scholar] [CrossRef]
- Pan, G.F.; Wang, Z.; Kong, D.Q.; Sun, T.W.; Zhai, H.; Tian, T.; Wang, Y.F.; Xing, R.G.; Zhang, B.W. Transparent, flame-retarded, self-healable, mechanically strong polyurethane elastomers: Enabled by the synthesis of phosphorus/nitrogen-containing oxime chain-extender. J. Appl. Polym. Sci. 2021, 139, 51598. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Mechanically robust self-healing and recyclable flame-retarded polyurethane elastomer based on thermoreversible crosslinking network and multiple hydrogen bonds. Chem. Eng. J. 2020, 391, 123544. [Google Scholar] [CrossRef]
- Du, X.; Jin, L.; Deng, S.; Zhou, M.; Du, Z.; Cheng, X.; Wang, H. Recyclable, Self-Healing, and Flame-Retardant Solid-Solid Phase Change Materials Based on Thermally Reversible Cross-Links for Sustainable Thermal Energy Storage. ACS Appl. Mater. Int. 2021, 13, 42991–43001. [Google Scholar] [CrossRef]
- Gu, L.; Ge, Z.; Huang, M.; Luo, Y. Halogen-free flame-retardant waterborne polyurethane with a novel cyclic structure of phosphorus−nitrogen synergistic flame retardant. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wang, C.-S.; Zhang, J.; Wang, H.; He, M.; Ding, L.; Zhao, W.-W. Simultaneously improving the fracture toughness and flame retardancy of soybean oil-based waterborne polyurethane coatings by phosphorus-nitrogen chain extender. Ind. Crops. Prod. 2021, 163, 113328. [Google Scholar] [CrossRef]
- Zhang, P.K.; He, Y.Z.; Tian, S.Q.; Fan, H.J.; Chen, Y.; Yan, J. Flame Retardancy, Mechanical, and Thermal Properties of Waterborne Polyurethane Conjugated With a Novel Phosphorous-Nitrogen Intumescent Flame Retardant. Polym. Compos. 2017, 38, 452–462. [Google Scholar] [CrossRef]
- Duan, N.; Sun, Z.; Ren, Y.; Liu, Z.; Liu, L.; Yan, F. Imidazolium-based ionic polyurethanes with high toughness, tunable healing efficiency and antibacterial activities. Polym. Chem. 2020, 11, 867–875. [Google Scholar] [CrossRef]
- Feng, X.; Li, G. Versatile Phosphate Diester-Based Flame Retardant Vitrimers via Catalyst-Free Mixed Transesterification. ACS Appl. Mater. Int. 2020, 12, 57486–57496. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Yuan, W.; Wang, B.; Zhu, J. Robust, Fire-Safe, Monomer-Recovery, Highly Malleable Thermosets from Renewable Bioresources. Macromolecules 2018, 51, 8001–8012. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Feng, Y.; Zhang, H.; Li, J.; Shi, X. Design of a self-healing and flame-retardant cyclotriphosphazene-based epoxy vitrimer. J. Mater. Sci. 2018, 53, 7030–7047. [Google Scholar] [CrossRef]
- Nie, H.; Schauser, N.S.; Self, J.L.; Tabassum, T.; Oh, S.; Geng, Z.; Jones, S.D.; Zayas, M.S.; Reynolds, V.G.; Chabinyc, M.L.; et al. Light-Switchable and Self-Healable Polymer Electrolytes Based on Dynamic Diarylethene and Metal-Ion Coordination. J. Am. Chem. Soc. 2021, 143, 1562–1569. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, L.; Yang, Q.; Huang, S.; Shi, H.; Long, Q.; Qian, B.; Liu, Z.; Guan, Q.; Liu, M.; et al. Self-healing polyurethane-elastomer with mechanical tunability for multiple biomedical applications in vivo. Nat. Commun. 2021, 12, 4395. [Google Scholar] [CrossRef]
- Celebi, F.P., O.; Aras, L.; Gündüz, G.; Akhmedov, I.M. Synthesis and Characterization of Water-Dispersed FlameRetardant Polyurethane Resin Using PhosphorusContaining Chain Extender. J. Appl. Polym. Sci. 2002, 91, 1314–1321. [Google Scholar] [CrossRef]
- Cui, J.G.; Liu, S.J.; Li, Q.X.; Chen, J.Q.; Tang, X.S.; Li, S.Q.; Zhong, S.Q.; Feng, W. A novel intrinsic flame-retardant waterborne poly(urethane) copolymers containing phosphorus-nitrogen. Fire Mater. 2022, 46, 443–449. [Google Scholar] [CrossRef]
- Gu, L. Preparation and application of P-N synergistic flame retardant polyester polyol. Polym. Mater. Sci. Eng. 2019, 35, 36–42. [Google Scholar]
- Wang, H.Y.; Jiang, P.P.; Zhang, P.B.; Zhao, H.H.; Zhao, M.Z.; Deng, J.N.; Cao, Z.L. Synthesis of polyols containing nitrogen-phosphorus from vegetable oil derivatives for polyurethane film applications. J Appl. Polym. Sci. 2021, 138, 50839. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Mu, X.W.; Wang, B.B.; Hu, W.Z.; Wang, J.L.; Zhou, F.; Ma, C.; Hu, Y.; Song, L. A novel phosphorous-containing polymeric compatibilizer: Effective reinforcement and flame retardancy in glass fiber reinforced polyamide 6 composites. Compos. Part B-Eng. 2021, 205, 108536. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Synthesis of a novel flame retardant based on DOPO derivatives and its application in waterborne polyurethane. RSC Adv. 2019, 9, 7411–7419. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, J.; Chen, X.; Hong, W.; Chen, Y.; Xiang, J.; Yan, J.; Fan, H. A halogen-free, flame retardant, waterborne polyurethane coating based on the synergistic effect of phosphorus and silicon. Prog. Org. Coat. 2021, 158, 106359. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, T.; Xu, Y.; Zhang, X. A phosphorus- and nitrogen-containing aromatic Schiff base derivative in waterborne polyurethane backbone for excellent flame retardant, UV-shielding and mechanical properties. Prog. Org. Coat. 2023, 182, 107631. [Google Scholar] [CrossRef]
- Dogan, M.; Dogan, S.D.; Savas, L.A.; Ozcelik, G.; Tayfun, U. Flame retardant effect of boron compounds in polymeric materials. Compos. Part B-Eng. 2021, 222, 109088. [Google Scholar] [CrossRef]
- El Khatib, W.; Youssef, B.; Bunel, C.; Mortaigne, B. Fireproofing of polyurethane elastomers by reactive organophosphonates. Polym. Int. 2003, 52, 146–152. [Google Scholar] [CrossRef]
- Li, X.; Bao, Q.; Chen, K.; He, R.; Liu, Y.; Wang, Q. Reprocessable Flame Retardant Silicone Polyurethane Based on Dynamic Phenylborate Ester Covalent and N−P−Si Synergistic Effect. ACS Sustain. Chem. Eng. 2022, 10, 14174–14184. [Google Scholar] [CrossRef]
- Li, Y.-J.; Chen, S.; Wu, F. Waterborne Polyurethane Polymer Electrolytes Containing Poly(ethylene glycol) and Polydimethylsiloxane in Soft Segments. Chem. Asian. J. 2014, 26, 5703–5708. [Google Scholar] [CrossRef]
- Liang, X.; Liu, C.; Chen, Y.; Yin, F.; Bao, D.; Zhou, G. Waterborne polyurethane composites flame retardancy based on the bi-DOPO derivatives and dangling poly(dimethylsiloxane) chains. Prog. Org. Coat. 2023, 177. [Google Scholar] [CrossRef]
- Feng, L.; Wang, W.; Song, B.; Zhu, X.; Wang, L.; Shao, R.; Li, T.; Pei, X.; Wang, L.; Qian, X.; et al. Synthesis of P, N and Si-containing waterborne polyurethane with excellent flame retardant, alkali resistance and flexibility via one-step synthetic approach. Prog. Org. Coat. 2023, 174, 107286. [Google Scholar] [CrossRef]
- Hu, C.; Li, J.; Pan, X.; Zeng, Y. Intrinsically flame-retardant vanillin-based PU networks with self-healing and reprocessing performances. Ind. Crops. Prod. 2023, 200, 116828. [Google Scholar] [CrossRef]
- Wu, J.Z.; Zhang, X.; Qin, Z.L.; Zhang, W.C.; Yang, R.J. Inorganic/organic phosphorus-based flame retardants synergistic flame retardant rigid polyurethane foam. Polym. Eng. Sci. 2023, 63, 1041–1049. [Google Scholar] [CrossRef]
- Gaan, S. Flame retardant flexible polyurethane foams from novel DOPOphosphonamidate additives. Polym. Degrad. Stab. 2015, 38, 83–93. [Google Scholar] [CrossRef]
- He, F.-M.; Liu, B.-W.; Chen, L.; Guo, D.-M.; Ding, X.-M.; Xiao, Y.-F.; Zhao, H.-B.; Li, W.-D.; Wang, Y.-Z. Novel polyamide 6 composites based on Schiff-base containing phosphonate oligomer: High flame retardancy, great processability and mechanical property. Compos Part A 2021, 146, 106423. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Rao, W.H.; Zhu, Z.M.; Wang, S.X.; Wang, T.; Tan, Y.; Liao, W.; Zhao, H.B.; Wang, Y.Z. A reactive phosphorus-containing polyol incorporated into flexible polyurethane foam: Self-extinguishing behavior and mechanism. Polym. Degrad. Stab. 2018, 153, 192–200. [Google Scholar] [CrossRef]
- Rao, W.H.; Xu, H.X.; Xu, Y.J.; Qi, M.; Liao, W.; Xu, S.M.; Wang, Y.Z. Persistently flame-retardant flexible polyurethane foams by a novel phosphorus-containing polyol. Chem. Eng. J. 2018, 343, 198–206. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Velencoso, M.M.; Rodríguez, J.F.; Serrano, Á.; Carrero, M.J.; Ramos, M.J. Synthesis of aminophosphonate polyols and polyurethane foams with improved fire retardant properties. J. Appl. Polym. Sci. 2019, 136, 47780. [Google Scholar] [CrossRef]
- Ren, H.; Sun, J.Z.; Wu, B.J.; Zhou, Q.Y. Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Shen, A.; Neisiany, R.E.; You, Z.; Zhu, M. Supertough spontaneously self-healing polymer based on septuple dynamic bonds integrated in one chemical group. Sci. China Chem. 2021, 65, 363–372. [Google Scholar] [CrossRef]
- Lu, J.-H.; Xu, Y.-J.; Chen, L.; Chen, J.-H.; He, J.-H.; Li, Z.; Li, S.-L.; Wang, Y.-Z. Facile fabrication of intrinsically fire-safety epoxy resin cured with phosphorus-containing transition metal complexes for flame retardation, smoke suppression, and latent curing behavior. Chem. Eng. J. 2022, 442, 136097. [Google Scholar] [CrossRef]
- Rabello, L.G.; Carlos da Conceição Ribeiro, R. A novel vermiculite/vegetable polyurethane resin-composite for thermal insulation eco-brick production. Compos. Part B-Eng. 2021, 221, 109035. [Google Scholar] [CrossRef]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame Retardancy of Bio-Based Polyurethanes: Opportunities and Challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef]
- Wendels, S.; Averous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Özşeker, A.; Karadeniz, K.; Fikret Yılmaz, R. Intrinsically Flame Retardant Polyurethane Prepared with Epoxidized Soybean Oil and Vinylphosphonic Acid. Croat. Chem. Acta 2018, 91, 589–597. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Flame Retardancy of Biobased Composites-Research Development. Materials 2020, 13, 5253. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Y.M.; Liu, B.Y.; Zhao, Q.; Qi, Y.X.; Wang, Y.M.; Sun, Z.Y.; Liu, B.J.; Zhang, N.N.; Hu, W.; et al. A novel phosphorus-containing lignin-based flame retardant and its application in polyurethane. Compos. Commun. 2020, 21, 100382. [Google Scholar] [CrossRef]
- Naiker, V.E.; Mestry, S.; Nirgude, T.; Gadgeel, A.; Mhaske, S.T. Recent developments in phosphorous-containing bio-based flame-retardant (FR) materials for coatings: An attentive review. J Coat. Technol. Res. 2022, 20, 113–139. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Lv, P.; Xie, S.; Sun, Q.; Chen, X.; He, Y. Flame-Retardant Solid Polymer Electrolyte Based on Phosphorus-Containing Polyurethane Acrylate/Succinonitrile for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 7199–7209. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.H.; Liu, J.Y.; Liu, X.Q.; Yang, X.; Jiang, X.L. A novel intrinsic flame-retardant and flexible polyurethane solid electrolyte for lithium batteries. Mater. Chem. Phys. 2022, 279, 125763. [Google Scholar] [CrossRef]
- Chen, J.; Rong, L.; Liu, X.Q.; Liu, J.Y.; Yang, X.; Jiang, X.L. Enhancement of flame retardancy of solid polymer electrolyte based on phosphorus-containing ionic liquid polyurethane membrane for safe lithium batteries. Polymer 2023, 269, 125759. [Google Scholar] [CrossRef]
- Xue, Y.; Lin, J.; Wan, T.; Luo, Y.; Ma, Z.; Zhou, Y.; Tuten, B.T.; Zhang, M.; Tao, X.; Song, P. Stretchable, Ultratough, and Intrinsically Self-Extinguishing Elastomers with Desirable Recyclability. Adv. Sci. 2023, 10, 2207268. [Google Scholar] [CrossRef]
- Kim, S.M.; Jeon, H.; Shin, S.H.; Park, S.A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, T.; Luo, Y.; Wang, Y.; Li, J.; Ye, T.; Guo, R.; Song, P.; Zhou, J.; Wang, H. Multifunctional polyurethane sponge coatings with excellent flame retardant, antibacterial, compressible, and recyclable properties. Compos. Part B-Eng. 2021, 215, 108785. [Google Scholar] [CrossRef]
- Guo, C.; Cao, Y.; Li, J.; Li, H.; Kumar Arumugam, S.; Oleksandr, S.; Chen, F. Solvent-free green synthesis of nonflammable and self-healing polymer film electrolytes for lithium metal batteries. Appl. Energy. 2022, 323, 119571. [Google Scholar] [CrossRef]
- Peng, J.; Xie, S.; Liu, T.; Wang, D.; Ou, R.; Guo, C.; Wang, Q.; Liu, Z. High-performance epoxy vitrimer with superior self-healing, shape-memory, flame retardancy, and antibacterial properties based on multifunctional curing agent. Compos. Part B-Eng. 2022, 242, 110109. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Zhou, W.; Liu, T.; Xu, P.; Puglia, D.; Kenny, J.M.; Ma, P. Design of inherent fire retarding and degradable bio-based epoxy vitrimer with excellent self-healing and mechanical reprocessability. Compos. Sci. Technol. 2022, 230, 109776. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, M.; Zuo, C.; Chen, G.; He, D.; Zhou, X.; Liu, C.; Xie, X.; Xue, Z. Flexible, Self-Healing, and Fire-Resistant Polymer Electrolytes Fabricated via Photopolymerization for All-Solid-State Lithium Metal Batteries. ACS Macro Lett. 2020, 9, 525–532. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Ma, S.; Xu, X.; Qiu, J.; Li, Q.; Wang, S.; Lu, N.; Ye, J.; Zhu, J. Phosphate-based covalent adaptable networks with recyclability and flame retardancy from bioresources. Eur. Polym. J. 2021, 144, 110236. [Google Scholar] [CrossRef]
- Markwart, J.C.; Battig, A.; Urbaniak, T.; Haag, K.; Koschek, K.; Schartel, B.; Wurm, F.R. Intrinsic flame retardant phosphonate-based vitrimers as a recyclable alternative for commodity polymers in composite materials. Polym. Chem. 2020, 11, 4933–4941. [Google Scholar] [CrossRef]
- Feng, X.; Fan, J.; Li, A.; Li, G. Multi-reusable thermoset with anomalous flame triggered shape memory effect. ACS Appl. Mater. Interfaces 2019, 11, 16075–16086. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Zhang, Q.; Lopez, J.; Wang, H.; Wu, H.C.; Niu, S.; Yan, H.; Wang, S.; Lei, T.; et al. Quadruple H-Bonding Cross-Linked Supramolecular Polymeric Materials as Substrates for Stretchable, Antitearing, and Self-Healable Thin Film Electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Li, Y.; Li, X.; Song, D.; Min, P.; Hu, C.; Zhang, H.B.; Koratkar, N.; Yu, Z.Z. Highly sensitive, reliable and flexible piezoresistive pressure sensors featuring polyurethane sponge coated with MXene sheets. J. Colloid. Int. Sci. 2019, 542, 54–62. [Google Scholar] [CrossRef]
- Liu, F.; Han, F.; Ling, L.; Li, J.; Zhao, S.; Zhao, T.; Liang, X.; Zhu, D.; Zhang, G.; Sun, R.; et al. An Omni-Healable and Highly Sensitive Capacitive Pressure Sensor with Microarray Structure. Chemistry 2018, 24, 16823–16832. [Google Scholar] [CrossRef]
- Ling, L.; Liu, F.; Li, J.; Zhang, G.; Sun, R.; Wong, C.-P. Self-Healable and Mechanically Reinforced Multidimensional-Carbon/Polyurethane Dielectric Nanocomposite Incorporates Various Functionalities for Capacitive Strain Sensor Applications. Macromol. Chem. Phys. 2018, 219, 1800369. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Gaan, S. Recent studies on the decomposition and strategies of smoke and toxicity suppression for polyurethane based materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef]
- Habibi, N.; Bagherifard, M.; Pourjavadi, A. Facile fabrication of flame-resistant, photothermal, and electrothermal polyurethane sponge: A promising sorbent for all-weather recovery of viscous crude oil spills from seawater. Colloid Surface A 2023, 656, 130401. [Google Scholar] [CrossRef]





















| Type | Representation | Advantage | Disadvantage |
|---|---|---|---|
| Polyether polyol | PEG | Hydrolytic stability↑, cost↓, flexibility↑ | Oxidative stability↓, strength↓, flammability↑, thermal stability↓, oxidative stability↓, cost↑ |
| PPG | |||
| PTMEG | Hydrolytic stability↑, strength↑ | ||
| Polyester polyol | Aliphatic polyester polyol | Oxidative stability↑, strength↑ | Hydrolytic stability↓ |
| Aromatic polyester polyol | Thermal stability↑, stiffness↑ | Flexibility↓ | |
| Polyolefin polyol | Polybutadiene Polyol | Low-temperature flexibility↑, solvent resistance↑ | Cost↑ |
| Type | Representation | Advantage | Disadvantage |
|---|---|---|---|
| Aromatic di-isocyanate | TDI | High reactivity, low production cost | Photo-oxidative instability |
| MDI | |||
| Aliphatic di-isocyanate | HDI | High chemical stability, excellent weather resistance | High production cost, low reactivity |
| IPDI | |||
| HMDI |
| HS | SS | Chain Extender | Quantity of P Component (wt. %) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | Tensile Strength (MPa) | Elongation at Break (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| HMDI | PPG | NPG | 0 | 278.1 | 1.46 | No | 18.2 | 16.4 | 264.1 | [60] |
| EPDD | 0.25 | 249.4 | 3.32 | V-0 | 26.6 | 14.0 | 402.2 | |||
| MDI | PCL | BDO | 0 | 344.9 | 2.67 | V-2 | 21 | 28.3 | 702 | [61] |
| HMCPP | 1.19 | 325.7 | 17.7 | V-0 | 31.8 | 6.07 | 1235 |
| HS | SS | Chain Extender | Content of P Component (wt.%) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | PHRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| IPDI | PPG | BDO | 0 | 272.0 | 0.69 | - | 17.8 | 727 | 93.8 | [55] |
| BPAMPP | 0.54 | 284.3 | 1.61 | - | 27.4 | 610 | 73.8 | |||
| IPDI | PBA | - | 0 | 165.1 | 2.14 | No | 19.3 | 812.3 | - | [56] |
| THPO | 0.44 | 263.9 | 4.37 | V-0 | 25 | 444.7 | - |
| Sample | Tensile Strength (Mpa) | Elongation at Break (%) | ||||
|---|---|---|---|---|---|---|
| Before Hydrolysis | After Hydrolysis | Retention Ratio (%) | Before Hydrolysis | After Hydrolysis | Retention Ratio (%) | |
| WPU | 16.4 | 15.4 | 93.9 | 264.1 | 244.4 | 92.5 |
| PWPU-3 a | 15.6 | 14.6 | 93.6 | 305.5 | 277 | 90.7 |
| PWPU-6 | 15.2 | 14.1 | 92.8 | 339.6 | 308 | 90.7 |
| PWPU-9 | 14.8 | 13.5 | 91.2 | 372.2 | 334.7 | 90 |
| PWPU-12 | 14.0 | 13.0 | 92.9 | 402.2 | 366.6 | 91.1 |
| HS | SS | Quantity of P Component (wt.%) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| MDI | PPO | 0 | 260 | 0 | - | 20 | 31 | - | 108 | [64] |
| 1.44 | 149 | 2.09 | - | 30 | 39 | - | 83.7 | |||
| MDI | OP | 0 | 304.7 | 3.8 | V-2 | 22.4 | 24 | 2232 | 132.2 | [65] |
| 9.5 | 212.7 | 24.37 | V-0 | 25.2 | 19 | 643 | 47.5 | |||
| MDI | PCEPEP-PO | 0 | 324.2 | 7.7 | - | 26.5 | 68 | 570.54 | 91.77 | [66] |
| 2.58 | 288.1 | 34.8 | - | 28.8 | 26 | 549.55 | 25.5 | |||
| MDI | FRPE | 0 | 323 | 0 | V-2 | 19 | 85 | 758 | 70 | [67] |
| 0.72 | 288 | 16 | V-0 | 24 | 101 | 664 | 53 |
| A | Sample | Tensile Strength (MPa) | Elongation at Break (%) | B | Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| PUE | 16.3 | 1016.1 | FR-PUE-28 b | 25.0 | 606.2 | ||
| FR-PUE-0.14 a | 16.2 | 904.4 | FR-PUE-34 b | 28.2 | 642.7 | ||
| FR-PUE-0.29 a | 17.6 | 791.6 | FR-PUE-37 b | 40.4 | 466.9 | ||
| FR-PUE-0.58 a | 20.7 | 693.2 | FR-PUE-40 b | 38.0 | 496.1 | ||
| FR-PUE-0.72 a | 25.0 | 605.8 | FR-PUE-90 b | 20.7 | 703.0 |
| Atmosphere | PU | T5% (°C) | T50% (°C) | T70% (°C) | Char Residue (wt.%) |
|---|---|---|---|---|---|
| N2 | REF | 304.7 | 319.7 | 424.7 | 7.28 |
| FPU-9.5 | 212.7 | 297.8 | 513.3 | 24.37 | |
| O2 | REF | 307.3 | 397.3 | 464.8 | 0.00 |
| FPU-9.5 | 217.2 | 459.2 | 665.7 | 30.14 |
| Soft Segment | Hard Segment | Mw (Da) | Hardness | T10% (°C) | Char Residue (wt. %) | LOI (%) | UL 94 | |
|---|---|---|---|---|---|---|---|---|
| EERPPU-1 | Phospho- polyol | TDI | 7907 | 80 | 225 | 10.09 | 29 | V-0 |
| EERPPU-2 | IPDI | 9016 | 75 | 265 | 8.56 | 27 | V-1 | |
| EERPPU-3 | HMDI | 7612 | 72 | 225 | 8.03 | 26 | V-1 | |
| EERPPU-4 | MDI | 9330 | 83 | 279 | 11.41 | 30 | V-0 |
| HS | SS | Chain Extender | Quantity of P/N Component (wt.%/wt.%) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | PHRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| IPDI | PPG | BDO | 0/0 | 274.3 | 0 | No | 19.5 | 1417.6 | 59.4 | [76] |
| BHAPE | 0.85/0.38 | 257.6 | 4.4 | V-2 | 25.5 | 810 | 45 | |||
| IPDI | PPG | PDNP | 0/0 | 261.2 | 0 | - | 19.5 | 866 | 61 | [73] |
| 1.61/0.73 | 244.9 | 1.62 | - | 26 | 705 | 46 | ||||
| HMDI | PPG | NPG | 0/0 | 276.9 | 0.71 | No | 18.5 | 1059 | 71.5 | [75] |
| PNMPD | 1.4/0.63 | 252.8 | 5.44 | V-0 | 27.2 | 586 | 43.6 | |||
| MDI | PTMEG | PSK-2 | 0/0 | 271 | 5.7 | V-2 | 18.7 | - | - | [77] |
| 0.87/3.2 | 330 | 8.7 | V-1 | 29.2 | - | - | ||||
| TDI | PPG | ODDP | 0/0 | 245.6 | 3.06 | V-2 | 25.4 | - | - | [80] |
| 4.13/3.73 | 208.2 | 18.18 | V-0 | 29.8 | - | - | ||||
| IPDI | PTMEG | BH | 0 | 318.4 | 0.2 | No | - | - | - | [81] |
| 1.12/0.5 | 289.9 | 2.57 | V-0 | 28.3 | - | - |
| HS | SS | Chain Extender | Quantity of P/N Component (wt.%/wt.%) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI(%) | Tensile Strength (MPa) | Elongation at Break (%) | PHRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HMDI | PPG | NPG a | 0/0 | 278.1 | 1.46 | No | 18.2 | 16.4 | 264.1 | - | - | [60] |
| EPPD | 0.25/0 | 249.4 | 3.32 | V-0 | 26.6 | 14.0 | 402.2 | - | - | |||
| HMDI | PPG | NPG a | 0/0 | 276.9 | 0.71 | No | 18.5 | 15 | 385.6 | 1059 | 71.5 | [75] |
| PNMPD | 1.4/0.63 | 252.8 | 5.44 | V-0 | 27.2 | 8.2 | 443.4 | 586 | 43.6 |
| HS | SS | Chain Extender | Quantity of P/N Component (wt. %/wt. %) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | Tensile Strength (MPa) | Elongation at Break (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| IPDI | PCDL | BDO | 0/0 | 274.3 | 0.74 | No | 18.6 | 15.3 | 347.2 | [82] |
| BSPB | 0.76/1.71 | 234.8 | 4.76 | V-0 | 27.3 | 26.4 | 264.3 |
| HS | SS | Quantity of P/N Component (wt.%/wt.%) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | HRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TDI | HO2-N2P4 | 0/0 | 266.2 | 5.71 | No | 19 | 491 | 132 | [90] |
| 6.1/1.4 | 192.6 | 35.86 | V-0 | 32 | 59 | 35 | |||
| TDI | JZP | 0/0 | - | 3.1 | No | 18 | 700 | - | [91] |
| 1.75/0.79 | - | 12.9 | V-0 | 28.6 | 400 | - | |||
| IPDI | PNP | 0/0 | 280 | 0.02 | No | 20.4 | 326 | 32.2 | [92] |
| 3.68/1.66 | 312 | 9.1 | V-0 | 25.5 | 105 | 21.7 |
| HS | SS | Chain Extender | Quantity of P/N Component (wt.%/wt.%) | T5% (°C) | Rchar (wt.%) | UL-94 | LOI (%) | PHRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| IPDI | PPG | BDO | 0/0 | 274.3 | 0 | No | 19.5 | 1417 | 59.4 | [76] |
| FRD | 0.79/0.25 | 264.5 | 5 | V-0 | 30.5 | 670 | 39.7 | |||
| IPDI | PPG | BDO | 0/0 | 280.1 | 1.21 | - | 18.4 | 976 | 64.48 | [94] |
| DOPO-DAM | 0.82/0.37 | 266.6 | 4.38 | - | 31 | 577 | 42.45 | |||
| IPDI | PCDL | BDO | 0/0 | 273 | 2.28 | No | 18.2 | 814 | 82 | [95] |
| PHID | 0.94/0.43 | 231 | 9.18 | V-0 | 25.1 | 448 | 62.5 |
| Samples a | Rchar (wt.%) | TTI (S) | THR (MJ/m2) | PHRR (kW/m2) | TSP (m2) | Tensile Strength (Mpa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| WPU | 2.3 | 31 | 82 | 814 | 5.3 | 13.4 | 429.6 |
| FR-WPU | 8.7 | 38 | 62.5 | 448 | 4.5 | 16.6 | 397.2 |
| SI-WPU | 3.9 | 39 | 66.6 | 550 | 4.7 | 12.8 | 463.5 |
| FR/SI-WPU | 12.6 | 44 | 31.6 | 159 | 1.9 | 15.9 | 435.6 |
| Name | Chemical Structure | Quantity of P Component (wt.%) | LOI (%) | UL-94 | PHRR (kW/m2) | THR (MJ/m2) | Ref. |
|---|---|---|---|---|---|---|---|
| HAMPP |  | 1.2 | 23.7 | V-0 | decrease 15.6% | decrease 27.5% | [106] |
| PPA-PO-polyol |  | 2 | - | - | decrease 68.6% | decrease 23.4% | [107] |
| PDEO |  | 2 | 23 | V-0 | No change | 62.4% | [108] |
| DMOP |  | 3.7 | 22.4 | V-0 | decrease 31.5% | decrease 43.8% | [109] |
| aminophosphonate polyether polyol |  | 3 | - | - | decrease 112.4% | decrease 125% | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Geng, Z.; Zhang, W.; He, J.; Yang, R. Strategy for Constructing Phosphorus-Based Flame-Retarded Polyurethane Elastomers for Advanced Performance in Long-Term. Polymers 2023, 15, 3711. https://doi.org/10.3390/polym15183711
Luo Y, Geng Z, Zhang W, He J, Yang R. Strategy for Constructing Phosphorus-Based Flame-Retarded Polyurethane Elastomers for Advanced Performance in Long-Term. Polymers. 2023; 15(18):3711. https://doi.org/10.3390/polym15183711
Chicago/Turabian StyleLuo, Yuxin, Zhishuai Geng, Wenchao Zhang, Jiyu He, and Rongjie Yang. 2023. "Strategy for Constructing Phosphorus-Based Flame-Retarded Polyurethane Elastomers for Advanced Performance in Long-Term" Polymers 15, no. 18: 3711. https://doi.org/10.3390/polym15183711
APA StyleLuo, Y., Geng, Z., Zhang, W., He, J., & Yang, R. (2023). Strategy for Constructing Phosphorus-Based Flame-Retarded Polyurethane Elastomers for Advanced Performance in Long-Term. Polymers, 15(18), 3711. https://doi.org/10.3390/polym15183711