Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
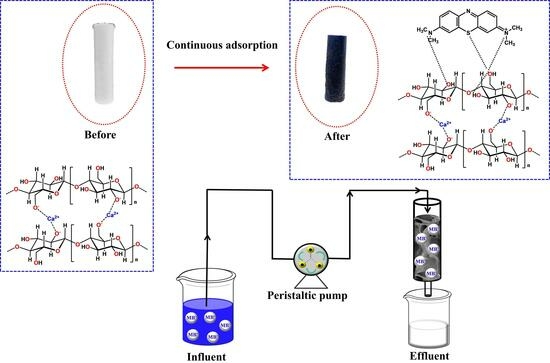
2.2. Monolithic Column Preparation and Characterization
2.3. Continuous-Flow Adsorption and Models
2.4. Influence of Temperature
2.5. Influence of Interference
2.6. Reusability of the Monolithic Cryogel
2.7. Real Sample Application
3. Results and Discussion
3.1. Characterization of the Cryogel Monolithic Column
3.2. Influence of Continuous-Flow Parameters
3.2.1. Initial MB Concentration
3.2.2. Flow Rate
3.2.3. Cryogel Column Height
3.2.4. Temperature
3.2.5. Interferences
3.3. Application of Dynamic Models
3.3.1. Application of the Adams–Bohart Model
3.3.2. Application of the Yoon–Nelson Model
3.4. Reusability of the Monolithic Cryogel
3.5. Real Sample Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auta, M.; Hameed, B.H. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. J. Chem. Eng. 2011, 171, 502–509. [Google Scholar] [CrossRef]
- Hu, X.S.; Liang, R.; Sun, G. Superadsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M.H. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Wang, P.; Cao, M.; Wang, C.; Ao, Y.; Hou, J.; Qian, J. Kinetics and thermodynamics of adsorption of methylene blue by a magnetic graphene-carbon nanotube composite. Appl. Surf. Sci. 2014, 290, 116–124. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Mahmoudi, A.; Amiri, S.; Darvishi, P.; Noori, E. Methylene blue removal using grape leaves waste: Optimization and modeling. Appl. Water Sci. 2022, 12, 112. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential candidates in wastewater treatment technology—A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Fang, K.; Deng, L.; Yin, J.; Yang, T.; Li, J.; He, W. Recent advances in starch-based magnetic adsorbents for the removal of contaminants from wastewater: A review. Int. J. Biol. Macromol. 2022, 218, 909–929. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Peng, X.; Sun, J.; Li, C.; Zhang, X.; Zhang, H.; Chen, J.; Zhou, X.; Zeng, H.; et al. Hydrogels for the removal of the methylene blue dye from wastewater: A review. Environ. Chem. Lett. 2022, 20, 2665–2685. [Google Scholar] [CrossRef]
- Khoo, P.S.; Ilyas, R.A.; Uda, M.N.A.; Hassan, S.A.; Nordin, A.H.; Norfarhana, A.S.; Ab Hamid, N.H.; Rani, M.S.A.; Abral, H.; Norrrahim, M.N.F.; et al. Starch-based polymer materials as advanced adsorbents for sustainable water treatment: Current status, challenges, and future perspectives. Polymers 2023, 15, 3114. [Google Scholar] [CrossRef] [PubMed]
- Taweekarn, T.; Wongniramaikul, W.; Boonkanon, C.; Phanrit, C.; Sriprom, W.; Limsakul, W.; Towanlong, W.; Phawachalotorn, C.; Choodum, A. Starch biocryogel for removal of methylene blue by batch adsorption. Polymers 2022, 14, 5543. [Google Scholar] [CrossRef]
- Dang, X.; Yu, Z.; Yang, M.; Woo, M.W.; Song, Y.; Wang, X.; Zhang, H. Sustainable electrochemical synthesis of natural starch-based biomass adsorbent with ultrahigh adsorption capacity for Cr(VI) and dyes removal. Sep. Purif. Technol. 2022, 288, 120668. [Google Scholar] [CrossRef]
- Srikaew, M.; Jumpapaeng, P.; Suwanakood, P.; Kaiyasuan, C.; Promarak, V.; Saengsuwan, S. Rapid synthesis and optimization of UV-photopolymerized cassava starch-based superabsorbent hydrogels as a biodegradable, low-cost, and effective adsorbent for MB removal. J. Ind. Eng. Chem. 2023, 118, 53–69. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Waeijen, H.A.; Berry, R.M.; Tam, K.C. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr. Polym. 2016, 136, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Mahamadi, C.; Mawere, E. Continuous flow biosorptive removal of methylene blue and crystal violet dyes using alginate–water hyacinth beads. Cogent Environ. Sci. 2019, 5, 1594513. [Google Scholar] [CrossRef]
- Kopsidas, O. Fixed-bed-column studies for methylene blue removal and recovery by untreated coffee residues. J. Environ. Sci. Health Part. A Environ. Sci. Eng. 2016, 5, 412–418. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, L.; Yan, H.; Li, H.; Jiang, Z.; Kan, X.; Yang, H.; Li, A.; Cheng, R. Removal of methylene blue from aqueous solutions by straw based adsorbent in a fixed-bed column. J. Chem. Eng. 2011, 173, 429–436. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Sun, T. Effective removal of methylene blue dye from water with nanocomposite ceramsites in a fixed-bed column. Environ. Technol. 2021, 42, 3807–3819. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Chang, J.S.; Govindwar, S.P. Fixed-bed decolorization of reactive blue 172 by Proteus vulgaris NCIM-2027 immobilized on Luffa cylindrica sponge. Int. Biodeterior. Biodegrad. 2011, 65, 494–503. [Google Scholar] [CrossRef]
- Malakhova, I.; Privar, Y.; Parotkina, Y.; Eliseikina, M.; Golikov, A.; Skatova, A.; Bratskaya, S. Supermacroporous monoliths based on polyethyleneimine: Fabrication and sorption properties under static and dynamic conditions. J. Environ. Chem. Eng. 2020, 8, 104395. [Google Scholar] [CrossRef]
- Kumari, H.J.; Krishnamoorthy, P.; Arumugam, T.K.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from wastewater by adsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.A.; Khan, A.Y. Selective adsorption of anionic dye from wastewater using polyethyleneimine based macroporous sponge: Batch and continuous studies. J. Hazard. Mater. 2022, 428, 128238. [Google Scholar] [CrossRef]
- Boonkanon, C.; Phatthanawiwat, K.; Chuenchom, L.; Lamthornkit, N.; Taweekarn, T.; Wongniramaikul, W.; Choodum, A. Preparation and characterization of calcium cross-linked starch monolithic cryogels and their application as cost-effective green filters. Polymers 2021, 13, 3975. [Google Scholar] [CrossRef]
- Phatthanawiwat, K.; Boonkanon, C.; Wongniramaikul, W.; Choodum, A. Catechin and curcumin nanoparticle-immobilized starch cryogels as green colorimetric sensors for on-site detection of iron. Sustain. Chem. Pharm. 2022, 29, 100782. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.E.; Morales-Barrera, L.; Cristiani-Urbina, E. Continuous biosorption of acid red 27 azo dye by Eichhornia crassipes leaves in a packed-bed column. Sci. Rep. 2021, 11, 18413. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Fixed-bed dynamic column adsorption study of methylene blue (MB) onto pine cone. Desalin. Water Treat. 2015, 55, 1026–1039. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Kapur, M.; Mondal, M.K. Design and model parameters estimation for fixed–bed column adsorption of Cu(II) and Ni(II) ions using magnet-ized saw dust. Desalin. Water Treat. 2016, 57, 12192–12203. [Google Scholar] [CrossRef]
- Ramirez, A.; Giraldo, S.; García-Nunez, J.; Flórez, E.; Acelas, N. Phos-phate removal from water using a hybrid material in a fixed-bed column. J. Water Process. Eng. 2018, 26, 131–137. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.; Poe, B.; McMillan, P.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 2004, 82, 742–748. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Res. Chem. Intermed. 2016, 42, 2343–2364. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Birgani, P.M.; Ranjbar, N.; Abdullah, R.C.; Wong, K.T.; Lee, G.; Ibrahim, S.; Park, C.; Yoon, Y.; Jang, M. An efficient and economical treatment for batik textile wastewater containing high levels of silicate and organic pollutants using a sequential process of acidification, magnesium oxide, and palm shell-based activated carbon application. J. Environ. Manag. 2016, 184, 229–239. [Google Scholar] [CrossRef]
- Wan Mohd Khalik, W.F.; Ho, L.N.; Ong, S.A.; Wong, Y.; Nik Yusoff, N.N.A.; Ridwan, F. Decolorization and mineralization of batik wastewater through solar photocatalytic process. Sains Malays. 2015, 44, 607–612. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H.T. Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. [Google Scholar] [CrossRef]
- Boonkanon, C.; Phatthanawiwat, K.; Wongniramaikul, W.; Choodum, A. Curcumin nanoparticle doped starch thin film as a green colorimetric sensor for detection of boron. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117351. [Google Scholar] [CrossRef]
- Choodum, A.; Lamthornkit, N.; Boonkanon, C.; Taweekarn, T.; Phatthanawiwat, K.; Sriprom, W.; Limsakul, W.; Chuenchom, L.; Wongniramaikul, W. Greener monolithic solid phase extraction biosorbent based on calcium cross-linked starch cryogel composite graphene oxide nanoparticles for benzo(a)pyrene analysis. Molecules 2021, 26, 6163. [Google Scholar] [CrossRef]
- Taweekarn, T.; Wongniramaikul, W.; Choodum, A. Removal and recovery of phosphate using a novel calcium silicate hydrate composite starch cryogel. J. Environ. Manag. 2022, 301, 113923. [Google Scholar] [CrossRef] [PubMed]
- Phawachalotorn, C.; Wongniramaikul, W.; Taweekarn, T.; Kleangklao, B.; Pisitaro, W.; Limsakul, W.; Sriprom, W.; Towanlong, W.; Choodum, A. Continuous phosphate removal and recovery using a calcium silicate hydrate composite monolithic cryogel column. Polymers 2023, 15, 539. [Google Scholar] [CrossRef] [PubMed]
- Wongniramaikul, W.; Limsakul, W.; Choodum, A. A biodegradable colorimetric film for rapid low-cost field determination of formaldehyde contamination by digital image colorimetry. Food Chem. 2018, 249, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.A.; Malik, M.A. Biogenic fabrication of ZnO nanoparticles using Trigonella foenum-graecum (Fenugreek) for proficient photocatalytic degradation of methylene blue under UV irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 16156–16173. [Google Scholar] [CrossRef]
- Dinh, V.P.; Huynh, T.D.T.; Le, H.M.; Nguyen, V.D.; Dao, V.A.; Hung, N.Q.; Tuyen, L.A.; Lee, S.; Yi, J.; Nguyen, T.D.; et al. Insight into the adsorption mechanisms of methylene blue and chromium(III) from aqueous solution onto pomelo fruit peel. RSC Adv. 2019, 9, 25847–25860. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, S.; Popa, M.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan–clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. J. Chem. Eng. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Nguyen Phuoc, H.; Le, Q.T.; Pham, T.C.T.; Le, T.T. Synthesis of glue-free NaA zeolite granules from natural kaolin for the adsorption of Pb(II) ions in aqueous solution using a fixed-bed column study. ACS Omega 2021, 6, 21024–21032. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.S. Highly Efficient Removal of Methylene Blue Dye from an aqueous solution using cellulose acetate nanofibrous membranes modified by polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Hameed, B.H. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Jawad, A.H.; Sabar, S. Adsorption characteristics and mechanistic study of immobilized chitosan-montmorillonite composite for methyl orange removal. J. Polym. Environ. 2020, 28, 1901–1913. [Google Scholar] [CrossRef]







| Flow Rate (mL·min−1) | H (cm) | C0 (mg·L−1) | Tb (min) | ttotal (min) | Veff (mL) | mtotal (mg) | qtotal (mg) | qe (mg·g−1) | RE a (%) | EBCT b (min) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5.0 | 7.5 | 25 | 370 | 660 | 3300 | 82.5 | 81.0 | 11.2 | 98.2 | 7.36 |
| 5.0 | 7.5 | 50 | 240 | 480 | 2400 | 120 | 117.7 | 16.2 | 98.0 | 7.36 |
| 5.0 | 7.5 | 100 | 150 | 360 | 1800 | 180 | 173.5 | 23.9 | 96.4 | 7.36 |
| 2.5 | 7.5 | 100 | 200 | 420 | 1050 | 105 | 103.6 | 14.4 | 98.7 | 14.72 |
| 5.0 | 7.5 | 100 | 150 | 360 | 1800 | 180 | 173.5 | 23.9 | 96.4 | 7.36 |
| 10.0 | 7.5 | 100 | 50 | 120 | 1200 | 120 | 113.6 | 15.7 | 94.7 | 3.68 |
| 5.0 | 2.5 | 100 | 30 | 90 | 450 | 45 | 42.5 | 18.9 | 94.4 | 2.45 |
| 5.0 | 5.0 | 100 | 120 | 180 | 900 | 90 | 86.7 | 19.4 | 96.4 | 4.91 |
| 5.0 | 7.5 | 100 | 150 | 360 | 1800 | 180 | 173.5 | 23.9 | 96.4 | 7.36 |
| Temperature (°C) | Tb (min) | Ttotal (min) | Veff (mL) | qtotal (mg) | mtotal (mg) | qe (mg g−1) | RE (%) |
|---|---|---|---|---|---|---|---|
| 25 | 150 | 360 | 1800 | 173.50 | 180 | 23.90 | 96.40 |
| 35 | 210 | 360 | 1800 | 179.41 | 180 | 25.30 | 99.67 |
| 45 | 260 | 360 | 1800 | 179.52 | 180 | 25.32 | 99.74 |
| Samples | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Tb (min) | Ttotal (min) | Veff (mL) | qtotal (mg) | mtotal (mg) | qe (mg g−1) | RE (%) | |
| MB (100 mg L−1) | 150 | 360 | 1800 | 179.6 | 180 | 24.9 | 99.8 |
| MB + CV a | 160 | 360 | 1800 | 179.9 | 180 | 25.4 | 99.9 |
| MB + metal ions b | 250 | 360 | 1800 | 179.8 | 180 | 25.2 | 99.9 |
| MB + urea + sodium sulphate c | 210 | 360 | 1800 | 179.8 | 180 | 25.1 | 99.9 |
| MB + sodium silicate d | 170 | 360 | 1800 | 178.21 | 180 | 24.6 | 99.0 |
| Parameters | Adams–Bohart Model | Yoon–Nelson | ||||
|---|---|---|---|---|---|---|
| KAB × 10−4 (L·mg−1·min−1) | N0 (mg·L−1) | R2 | KYN (min−1) | τ (min) | R2 | |
| Initial conc. (mg L−1) | ||||||
| 25 | 4.76 | 1906 | 0.8635 | 0.0191 | 464.22 | 0.9695 |
| 50 | 3.30 | 3825 | 0.9265 | 0.0247 | 290.98 | 0.9670 |
| 100 | 1.66 | 4175 | 0.8502 | 0.0282 | 233.35 | 0.9654 |
| Flow rate (mL min−1) | ||||||
| 2.5 | 1.61 | 2402 | 0.8652 | 0.0333 | 257.62 | 0.9564 |
| 5.0 | 1.66 | 4175 | 0.8502 | 0.0282 | 233.35 | 0.9654 |
| 10.0 | 3.23 | 4790 | 0.7525 | 0.0747 | 70.68 | 0.9393 |
| Column height (cm) | ||||||
| 2.5 | 4.42 | 3318 | 0.9514 | 0.1194 | 51.24 | 0.9265 |
| 5.0 | 5.13 | 3336 | 0.9003 | 0.0899 | 136.34 | 0.9753 |
| 7.5 | 1.66 | 4175 | 0.8502 | 0.0282 | 233.35 | 0.9654 |
| Real sample | 1.79 | 196.6 | 0.6338 | 0.0283 | 262.44 | 0.9604 |
| Time of Use/Sample | Flow Rate (mL min−1) | H (cm) | C0 (mg L−1) | Tb (min) | ttotal (min) | Veff (mL) | mtotal (mg) | qtotal (mg) | qe (mg g−1) | RE a (%) | EBCT b (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.0 | 7.5 | 100 | 150 | 360 | 1800 | 180 | 179.6 | 24.9 | 99.8 | 7.36 |
| 2 | 5.0 | 7.5 | 100 | 30 | 180 | 900 | 90 | 80.2 | 8.7 | 89.1 | 7.36 |
| 3 | 5.0 | 7.5 | 100 | 10 | 90 | 450 | 45 | 28.2 | 2.7 | 62.7 | 7.36 |
| RW c | 5.0 | 7.5 | 3.6 | 200 | 480 | 2400 | 8.59 | 8.57 | 1.19 | 99.7 | 7.36 |
| Adsorbent | Initial Concentration (mg·L−1) | Flow Rate (mL·min−1) | Bed Height (cm) | qtotal (mg) | Reusability (Cycles) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Cellulose nanocrystal–alginate hydrogel beads | 250 | 4.17 | 7.4 | 19.37 | 5 | - | [16] |
| Alginate–water hyacinth beads | 20 | 1.5 | 2.5 | - | 3 | - | [17] |
| Pine cone | 70 | 12 | 10 | 147 | - | - | [30] |
| Chitosan–clay composite | 50 | 5.0 | 3.6 | 58.395 | - | - | [48] |
| Monolithic cryogel based on starch | 100 | 5.0 | 7.5 | 179.6 | 3 | ✓ | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taweekarn, T.; Wongniramaikul, W.; Sriprom, W.; Limsakul, W.; Choodum, A. Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column. Polymers 2023, 15, 3989. https://doi.org/10.3390/polym15193989
Taweekarn T, Wongniramaikul W, Sriprom W, Limsakul W, Choodum A. Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column. Polymers. 2023; 15(19):3989. https://doi.org/10.3390/polym15193989
Chicago/Turabian StyleTaweekarn, Tarawee, Worawit Wongniramaikul, Wilasinee Sriprom, Wadcharawadee Limsakul, and Aree Choodum. 2023. "Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column" Polymers 15, no. 19: 3989. https://doi.org/10.3390/polym15193989
APA StyleTaweekarn, T., Wongniramaikul, W., Sriprom, W., Limsakul, W., & Choodum, A. (2023). Continuous-Flow System for Methylene Blue Removal Using a Green and Cost-Effective Starch Single-Rod Column. Polymers, 15(19), 3989. https://doi.org/10.3390/polym15193989