Characterization of Cellulose Fiber Derived from Hemp and Polyvinyl Alcohol-Based Composite Hydrogel as a Scaffold Material
Abstract
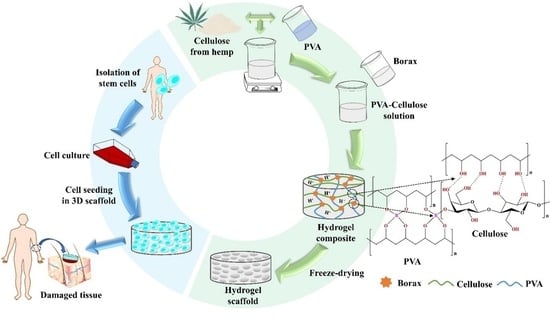
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methodology
2.2.1. Extraction and Purification of Cellulose Fiber
2.2.2. Extraction of Cellulose Nanocrystals (CNCs) with Sulfuric Acid Hydrolysis
2.2.3. Preparation of Cellulose Nanocrystal and Polyvinyl Alcohol-Based Composite Hydrogel
2.2.4. Swelling Behavior
2.2.5. Gel Fraction
2.2.6. Cytotoxic Test
2.3. Instruments
2.3.1. Fourier-Transform Infrared Spectroscopy
2.3.2. X-ray Diffraction
2.3.3. Scanning Electron Microscopy
2.3.4. Thermogravimetric Analysis
2.3.5. Differential Scanning Calorimetry
3. Results and Discussion
3.1. Characterization of Cellulose Nanocrystals from Hemp-Based Fiber
3.2. Fabrication of Cellulose Derived from Hemp and Polyvinyl Alcohol-Based Hydrogel Composite
3.3. Preliminary Investigation as a Biomaterial
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nature Nanotechnology. Nanomaterials definition matters. Nat. Nanotechnol. 2019, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; de Santiago, G.T. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yu, Y.; Zu, Y. Self-healing hydrogels based on the Knoevenagel condensation reaction for wound healing. Biomed. Technol. 2023, 2, 70–76. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Pisitsak, P.; Ummartyotin, S.; Narain, R. Polysaccharides for Medical Technology: Properties and Applications. Macromol. Biosci. 2022, 23, e2200372. [Google Scholar] [CrossRef]
- Misra, M.; Vivekanandhan, S.; Mohanty, A.K.; Denault, J. Nanotechnologies for Agricultural Bioproducts. In Comprehensive Biotechnology; National Research Council Canada: Ottawa, ON, Canada, 2011; pp. 119–127. [Google Scholar]
- Rukmanikrishnan, B.; Lee, J. Anti-freezing and thermally self-healing polymer composite comprising polyvinyl alcohol, polyethylene oxide, and sodium carboxymethyl cellulose. Eur. Polym. J. 2021, 154, 110565. [Google Scholar] [CrossRef]
- Revati, R.; Majid, M.A.; Ridzuan, M.; Mamat, N.; Cheng, E.; Alshahrani, H.A. In vitro biodegradation, cytotoxicity, and biocompatibility of polylactic acid/napier cellulose nanofiber scaffold composites. Int. J. Biol. Macromol. 2022, 223 Pt A, 479–489. [Google Scholar] [CrossRef]
- Doustdar, F.; Olad, A.; Ghorbani, M. Effect of glutaraldehyde and calcium chloride as different crosslinking agents on the characteristics of chitosan/cellulose nanocrystals scaffold. Int. J. Biol. Macromol. 2022, 208, 912–924. [Google Scholar] [CrossRef]
- Doostan, M.; Doostan, M.; Mohammadi, P.; Khoshnevisan, K.; Maleki, H. Wound healing promotion by flaxseed extract-loaded polyvinyl alcohol/chitosan nanofibrous scaffolds. Int. J. Biol. Macromol. 2022, 228, 506–516. [Google Scholar] [CrossRef]
- Sriwong, C.; Boonrungsiman, S.; Sukyai, P. Sugarcane bagasse cellulose-based scaffolds incorporated hydroxyapatite for promoting proliferation, adhesion and differentiation of osteoblasts. Ind. Crops Prod. 2023, 192, 115979. [Google Scholar] [CrossRef]
- Zhao, T.; Li, B.; Nie, K.; Ben, H.; Yang, X.; Zhang, Y.; Han, G.; Jiang, W. A novel cascade glycolic acid pretreatment-alkali degumming method for producing hemp fiber. Ind. Crops Prod. 2023, 195, 116424. [Google Scholar] [CrossRef]
- Sakwises, L.; Rodthongkum, N.; Ummartyotin, S. SnO2- and bacterial-cellulose nanofiber-based composites as a novel platform for nickel-ion detection. J. Mol. Liq. 2017, 248, 246–252. [Google Scholar] [CrossRef]
- Leong, S.L.; Tiong, S.I.X.; Siva, S.P.; Ahamed, F.; Chan, C.-H.; Lee, C.L.; Chew, I.M.L.; Ho, Y.K. Morphological control of cellulose nanocrystals via sulfuric acid hydrolysis based on sustainability considerations: An overview of the governing factors and potential challenges. J. Environ. Chem. Eng. 2022, 10, 108145. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Ikhuoria, E.; Omorogbe, S.; Agbonlahor, O.; Etiuma, R. Nanocellulose Crystals from Coir Fibre for Template Application. Am. Chem. Sci. J. 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Nacos, M.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.; Christakopoulos, P.; Polissiou, M. Kenaf xylan—A source of biologically active acidic oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Biopolymer-based materials from polysaccharides: Properties, processing, characterization and sorption applications. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Ramaswamy, V.; Vimalathithan, R.; Ponnusamy, V. Synthesis and characterization of BaSO4 nano particles using micro emulsion technique. Adv. Appl. Sci. Res. 2010, 1, 197–204. [Google Scholar]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Zhou, D. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef]
- Kassab, Z.; Abdellaoui, Y.; Salim, M.H.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Micro- and nano-celluloses derived from hemp stalks and their effect as polymer reinforcing materials. Carbohydr. Polym. 2020, 245, 116506. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Han, J.; Lei, T.; Wu, Q. High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef]
- Bian, H.; Jiao, L.; Wang, R.; Wang, X.; Zhu, W.; Dai, H. Lignin nanoparticles as nano-spacers for tuning the viscoelasticity of cellulose nanofibril reinforced polyvinyl alcohol-borax hydrogel. Eur. Polym. J. 2018, 107, 267–274. [Google Scholar] [CrossRef]
- Ge, G.; Yuan, W.; Zhao, W.; Lu, Y.; Zhang, Y.; Wang, W.; Chen, P.; Huang, W.; Si, W.; Dong, X. Highly stretchable and autonomously healable epidermal sensor based on multi-functional hydrogel frameworks. J. Mater. Chem. A 2019, 7, 5949–5956. [Google Scholar] [CrossRef]
- Ahmad, S.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Noor, T.; Hou, H.; Azhar, O.; Sher, E.K. Polyvinyl alcohol and aminated cellulose nanocrystal membranes with improved interfacial compatibility for environmental applications. Environ. Res. 2022, 214 Pt 1, 113793. [Google Scholar] [CrossRef] [PubMed]
- Azhar, O.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Kakar, S.J.; Shahid, M. Cellulose acetate-polyvinyl alcohol blend hemodialysis membranes integrated with dialysis performance and high biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112127. [Google Scholar] [CrossRef]
- Ogunjobi, J.K.; Adewale, A.I.; Adeyemi, S.A. Cellulose nanocrystals from Siam weed: Synthesis and physicochemical characterization. Heliyon 2023, 9, e13104. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Muthoka, R.M.; Kim, J. Electroactive Hydrogels Made with Polyvinyl Alcohol/Cellulose Nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Fernández-García, M.P.; Ventura, J.; Araújo, J.P.; Romano, A.; Lindman, B. Unusual extraction and characterization of nanocrystalline cellulose from cellulose derivatives. J. Mol. Liq. 2015, 210, 106–112. [Google Scholar] [CrossRef]
- de Lima, G.G.; Ferreira, B.D.; Matos, M.; Pereira, B.L.; Nugent, M.J.D.; Hansel, F.A.; Magalhaes, W.L.E. Effect of cellulose size-concentration on the structure of polyvinyl alcohol hydrogels. Carbohydr. Polym. 2020, 245, 116612. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, J.; Fan, X.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Improved mechanical and antibacterial properties of polyvinyl alcohol composite films using quaternized cellulose nanocrystals as nanofillers. Compos. Sci. Technol. 2023, 232, 109885. [Google Scholar] [CrossRef]
- Al Tawil, E.; Monnier, A.; Nguyen, Q.T.; Deschrevel, B. Microarchitecture of poly(lactic acid) membranes with an interconnected network of macropores and micropores influences cell behavior. Eur. Polym. J. 2018, 105, 370–388. [Google Scholar] [CrossRef]
- Mat-Shayut, M.S.; Abdullah, M.Z.; Megat-Yuso, P.S.M. Water Absorption Properties and Morphology of Polypropylene/ Polycarbonate/Polypropylene-graft-Maleic Anhydride Blends. Asian J. Sci. Res. 2013, 6, 167–176. [Google Scholar] [CrossRef]
- Gan, Y. Effect of Interface Structure on Mechanical Properties of Advanced Composite Materials. Int. J. Mol. Sci. 2009, 10, 5115–5134. [Google Scholar] [CrossRef]
- Mamouri, K.S.; Rahaiee, S.; Zare, M.; Kenari, M.N.; Mirzakhani, N. Physicochemical and thermal characterization, and evaluation of a bacterial cellulose/Barhang gum-based dressing for wound healing. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124660. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fibre. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef]
- Veeramachineni, A.K.; Sathasivam, T.; Muniyandy, S.; Janarthanan, P.; Langford, S.J.; Yan, L.Y. Optimizing Extraction of Cellulose and Synthesizing Pharmaceutical Grade Carboxymethyl Sago Cellulose from Malaysian Sago Pulp. Appl. Sci. 2016, 6, 170. [Google Scholar] [CrossRef]
- Nosrati, H.; Khouy, R.A.; Nosrati, A.; Khodaei, M.; Banitalebi-Dehkordi, M.; Ashrafi-Dehkordi, K.; Sanami, S.; Alizadeh, Z. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J. Nanobiotechnol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N-succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- Chami Khazraji, A.; Robert, S. Interaction Effects between Cellulose and Water in Nanocrystalline and Amorphous Regions: A Novel Approach Using Molecular Modeling. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Sethi, V.; Kaur, M.; Thakur, A.; Rishi, P.; Kaushik, A. Unravelling the role of hemp straw derived cellulose in CMC/PVA hydrogel for sustained release of fluoroquinolone antibiotic. Int. J. Biol. Macromol. 2022, 222 Pt A, 844–855. [Google Scholar] [CrossRef]
- Aswathy, S.H.; NarendraKumar, U.; Manjubala, I. Physicochemical Properties of Cellulose-Based Hydrogel for Biomedical Applications. Polymers 2022, 14, 4669. [Google Scholar] [CrossRef] [PubMed]
- Zandraa, O.; Ngwabebhoh, F.A.; Patwa, R.; Nguyen, H.T.; Motiei, M.; Saha, N.; Saha, T.; Saha, P. Development of dual crosslinked mumio-based hydrogel dressing for wound healing application: Physico-chemistry and antimicrobial activity. Int. J. Pharm. 2021, 607, 120952. [Google Scholar] [CrossRef] [PubMed]
- Seera, S.D.K.; Kundu, D.; Banerjee, T. Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: Freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose 2020, 27, 6521–6535. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Rao, Z.; Dong, Y.; Liu, J.; Zheng, X.; Pei, Y.; Tang, K. Genipin-crosslinked gelatin-based composite hydrogels reinforced with amino-functionalized microfibrillated cellulose. Int. J. Biol. Macromol. 2022, 222 Pt B, 3155–3167. [Google Scholar] [CrossRef]
- Dick, M.K.; Miao, J.H.; Limaiem, F. Histology, fibroblast. In StatPearls [Internet]; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]









| Sample | Gel Fraction (%) | |
|---|---|---|
| DI | PBS | |
| Pristine PVA | 68.389 ± 1.176 | 85.969 ± 2.452 |
| 1% CNC/PVA | 66.022 ± 6.022 | 87.235 ± 3.192 |
| 3% CNC/PVA | 64.980 ± 3.442 | 90.734 ± 6.040 |
| 5% CNC/PVA | 59.076 ± 3.424 | 88.531 ± 2.123 |
| 7% CNC/PVA | 64.906 ± 2.115 | 85.892 ± 2.021 |
| 10% CNC/PVA | 62.653 ± 1.115 | 85.637 ± 1.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promdontree, P.; Kheolamai, P.; Ounkaew, A.; Narain, R.; Ummartyotin, S. Characterization of Cellulose Fiber Derived from Hemp and Polyvinyl Alcohol-Based Composite Hydrogel as a Scaffold Material. Polymers 2023, 15, 4098. https://doi.org/10.3390/polym15204098
Promdontree P, Kheolamai P, Ounkaew A, Narain R, Ummartyotin S. Characterization of Cellulose Fiber Derived from Hemp and Polyvinyl Alcohol-Based Composite Hydrogel as a Scaffold Material. Polymers. 2023; 15(20):4098. https://doi.org/10.3390/polym15204098
Chicago/Turabian StylePromdontree, Praewa, Pakpoom Kheolamai, Artjima Ounkaew, Ravin Narain, and Sarute Ummartyotin. 2023. "Characterization of Cellulose Fiber Derived from Hemp and Polyvinyl Alcohol-Based Composite Hydrogel as a Scaffold Material" Polymers 15, no. 20: 4098. https://doi.org/10.3390/polym15204098
APA StylePromdontree, P., Kheolamai, P., Ounkaew, A., Narain, R., & Ummartyotin, S. (2023). Characterization of Cellulose Fiber Derived from Hemp and Polyvinyl Alcohol-Based Composite Hydrogel as a Scaffold Material. Polymers, 15(20), 4098. https://doi.org/10.3390/polym15204098