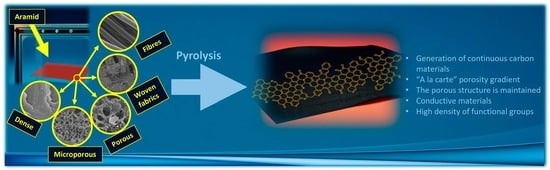
Crafting and Analyzing Multi-Structured Aramid Materials and Their Pyrolytic Transformations: A Comprehensive Exploration
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation and Methods
2.3. Preparation of GP-Aramids
2.4. Pyrolysis of Aramids
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinohara, Y. Functionally Graded Materials. In Handbook of Advanced Ceramics: Materials, Applications, Processing, and Properties, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1179–1187. [Google Scholar] [CrossRef]
- Levashov, E.A.; Gotman, I. Functionally Graded Materials. In Concise Encyclopedia of Self-Propagating High-Temperature Synthesis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 135–137. [Google Scholar] [CrossRef]
- Birman, V.; Byrd, L.W. Modeling and Analysis of Functionally Graded Materials and Structures. Appl. Mech. Rev. 2007, 60, 195–216. [Google Scholar] [CrossRef]
- Kieback, B.; Neubrand, A.; Riedel, H. Processing Techniques for Functionally Graded Materials. Mater. Sci. Eng. A 2003, 362, 81–106. [Google Scholar] [CrossRef]
- Miao, X.; Sun, D. Graded/Gradient Porous Biomaterials. Materials 2009, 3, 26–47. [Google Scholar] [CrossRef]
- Saleh, B.; Jiang, J.; Fathi, R.; Al-hababi, T.; Xu, Q.; Wang, L.; Song, D.; Ma, A. 30 Years of Functionally Graded Materials: An Overview of Manufacturing Methods, Applications and Future Challenges. Compos. Part B Eng. 2020, 201, 108376. [Google Scholar] [CrossRef]
- Neubrand, A.; Rödel, J. Gradient Materials: An Overview of a Novel Concept. Int. J. Mater. Res. 1997, 88, 358–371. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, Y.; Du, M.; Xie, Z. 316L Stainless Steel with Gradient Porosity Fabricated by Selective Laser Melting. J. Mater. Eng. Perform. 2010, 19, 666–671. [Google Scholar] [CrossRef]
- Fousová, M.; Vojtěch, D.; Kubásek, J.; Jablonská, E.; Fojt, J. Promising Characteristics of Gradient Porosity Ti-6Al-4V Alloy Prepared by SLM Process. J. Mech. Behav. Biomed. Mater. 2017, 69, 368–376. [Google Scholar] [CrossRef]
- Zhang, W.; Lv, J.; Wang, B.; Zhang, L.; Zhong, Y.; Sui, X.; Xu, H.; Mao, Z. Robust, Floatable, Steam Generator Based on the Graded Porous Polyimide Film for Efficient Solar Desalination. Polym. Adv. Technol. 2021, 32, 3436–3445. [Google Scholar] [CrossRef]
- Täuber, K.; Lepenies, B.; Yuan, J. Polyvinylpyridinium-Type Gradient Porous Membranes: Synthesis, Actuation and Intrinsic Cell Growth Inhibition. Polym. Chem. 2015, 6, 4855–4858. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, G.; Li, P.; Xue, F.; Chen, Z.; Zheng, H.; Ding, R.; Xiong, J.; Yan, Q.; Xu, L.; et al. Gradient In-Plane Oriented Porous Carbon Inspired by Fabrication of Toasts for Elegant EMI Shielding Performance. Carbon N. Y. 2023, 207, 136–143. [Google Scholar] [CrossRef]
- Bahner, J.; Hug, N.; Polarz, S. Anisotropic Magnetism in Gradient Porous Carbon Composite Aerogels. J. Carbon Res. 2021, 7, 22. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Yang, C.; Dang, B.; Li, C.; Sun, Q. A Multilevel Gradient Structural Carbon Derived from Naturally Preprocessed Biomass. Carbon N. Y. 2020, 168, 624–632. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Chernenko, D.N.; Kulichikhin, V.G. Graphitized Carbon Fibers Based on Lyocell Precursors. IOP Conf. Ser. Earth Environ. Sci. 2019, 316, 8–15. [Google Scholar] [CrossRef]
- Yuan, W.; Hu, J.; Zhou, B.; Deng, J.; Zhang, Z.; Tang, Y. Using Woven Carbon Fiber Fabric to Construct Gradient Porous Structure for Passive Direct Methanol Fuel Cells. Funct. Mater. Lett. 2015, 8, 1550009. [Google Scholar] [CrossRef]
- Nomura, K.; Nishihara, H.; Kobayashi, N.; Asada, T.; Kyotani, T. 4.4 V Supercapacitors Based on Super-Stable Mesoporous Carbon Sheet Made of Edge-Free Graphene Walls. Energy Environ. Sci. 2019, 12, 1542–1549. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring Porosity in Carbon Materials for Supercapacitor Applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Lei, H.; Wang, C.; Zhao, Y.; Huo, E.; Lin, X.; Zhang, Q.; Qian, M.; Mateo, W.; et al. Synthesis of Graphene-like Carbon from Biomass Pyrolysis and Its Applications. Chem. Eng. J. 2020, 399, 125808. [Google Scholar] [CrossRef]
- Gu, D.; Zhou, Y.; Ma, R.; Wang, F.; Liu, Q.; Wang, J. Facile Synthesis of N-Doped Graphene-Like Carbon Nanoflakes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction. Nano-Micro Lett. 2018, 10, 29. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, J.; Cao, X.; Abbott, J.; Jin, C.; Wang, H.; Strasser, P.; Yang, R.; Chen, X.; Wu, G. N-, P-, and S-Doped Graphene-like Carbon Catalysts Derived from Onium Salts with Enhanced Oxygen Chemisorption for Zn-Air Battery Cathodes. Appl. Catal. B Environ. 2019, 241, 442–451. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.; Chen, D.; Ma, W.; Wang, Y.; Xu, P.; Ma, J.; Zhao, H.; Han, X. Electromagnetic Functionalized Co/C Composites by in Situ Pyrolysis of Metal-Organic Frameworks (ZIF-67). J. Alloys Compd. 2016, 681, 384–393. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Fu, Q.; Chen, Y.; Dong, J.; Chen, W.; Li, Z.; Wang, Y.; Gu, L.; He, W.; et al. Confined Pyrolysis within Metal-Organic Frameworks to Form Uniform Ru3 Clusters for Efficient Oxidation of Alcohols. J. Am. Chem. Soc. 2017, 139, 9795–9798. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Lu, Y.; Wang, Y.; Wu, M.; Lou, X.W.D. Porous Iron–Cobalt Alloy/Nitrogen-Doped Carbon Cages Synthesized via Pyrolysis of Complex Metal–Organic Framework Hybrids for Oxygen Reduction. Adv. Funct. Mater. 2018, 28, 1706738. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Chen, H.; Liu, Y.; Yang, D.; Li, H. Graphene-like Porous Carbon Nanosheets Derived from Salvia Splendens for High-Rate Performance Supercapacitors. J. Power Sources 2018, 397, 1–10. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From Coconut Shell to Porous Graphene-like Nanosheets for High-Power Supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C. ping 3D Nitrogen-Doped Graphene Prepared by Pyrolysis of Graphene Oxide with Polypyrrole for Electrocatalysis of Oxygen Reduction Reaction. Nano Energy 2013, 2, 241–248. [Google Scholar] [CrossRef]
- Andronescu, C.; Barwe, S.; Ventosa, E.; Masa, J.; Vasile, E.; Konkena, B.; Möller, S.; Schuhmann, W. Powder Catalyst Fixation for Post-Electrolysis Structural Characterization of NiFe Layered Double Hydroxide Based Oxygen Evolution Reaction Electrocatalysts. Angew. Chem.-Int. Ed. 2017, 56, 11258–11262. [Google Scholar] [CrossRef]
- Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Studies on Pyrolysis of Nomex Polyaramid Fibers. J. Anal. Appl. Pyrolysis 2001, 58–59, 105–115. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, X.; Liang, G. Research on Pyrolysis Process of Kevlar Fibers with Thermogravimetric Analysis Coupled and Fourier Transform Infrared Spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 1374–1377. [Google Scholar]
- Gunathilake, S.S.; Huang, P.; Bhatt, M.P.; Rainbolt, E.A.; Stefan, M.C.; Biewer, M.C. Nitrogen Containing Graphene-like Structures from Pyrolysis of Pyrimidine Polymers for Polymer/Graphene Hybrid Field Effect Transistors. RSC Adv. 2014, 4, 41997–42001. [Google Scholar] [CrossRef]
- Gottlieb, E.; Matyjaszewski, K.; Kowalewski, T. Polymer-Based Synthetic Routes to Carbon-Based Metal-Free Catalysts. Adv. Mater. 2019, 31, 1804626. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent Progress on Nitrogen/Carbon Structures Designed for Use in Energy and Sustainability Applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Choma, J.; Osuchowski, L.; Marszewski, M.; Dziura, A.; Jaroniec, M. Developing Microporosity in Kevlar®-Derived Carbon Fibers by CO2 Activation for CO2 Adsorption. J. CO2 Util. 2016, 16, 17–22. [Google Scholar] [CrossRef]
- Karthik, D.; Baheti, V.; Militky, J.; Naeem, M.S.; Tunakova, V.; Ali, A. Activated Carbon Derived from Carbonization of Kevlar Waste Materials: A Novel Single Stage Method. Materials 2021, 14, 6433. [Google Scholar] [CrossRef] [PubMed]
- Karacan, I.; Erzurumluoğlu, L. The Effect of Carbonization Temperature on the Structure and Properties of Carbon Fibers Prepared from Poly(m-Phenylene Isophthalamide) Precursor. Fibers Polym. 2015, 16, 1629–1645. [Google Scholar] [CrossRef]
- Mosquera, M.E.G.; Jamond, M.; Martínez-Alonso, A.; Tascón, J.M.D. Thermal Transformations of Kevlar Aramid Fibers during Pyrolysis: Infrared and Thermal Analysis Studies. Chem. Mater. 1994, 6, 1918–1924. [Google Scholar] [CrossRef]
- Conte, G.; Stelitano, S.; Policicchio, A.; Minuto, F.D.; Lazzaroli, V.; Galiano, F.; Agostino, R.G. Assessment of Activated Carbon Fibers from Commercial Kevlar® as Nanostructured Material for Gas Storage: Effect of Activation Procedure and Adsorption of CO2 and CH4. J. Anal. Appl. Pyrolysis 2020, 152, 104974. [Google Scholar] [CrossRef]
- Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M. Activated Carbon Fibers from Nomex by Chemical Activation with Phosphoric Acid. Carbon N. Y. 2004, 42, 1419–1426. [Google Scholar] [CrossRef]
- Pascual, B.S.; Trigo-López, M.; Reglero Ruiz, J.A.; Pablos, J.L.; Bertolín, J.C.; Represa, C.; Cuevas, J.V.; García, F.C.; García, J.M. Porous Aromatic Polyamides the Easy and Green Way. Eur. Polym. J. 2019, 116, 91–98. [Google Scholar] [CrossRef]
- Rubio-Aguinaga, A.; Reglero-Ruiz, J.A.; Muñoz, A.; García, F.C.; García, J.M.; Trigo-López, M. Preparation of Low-Density High-Performance Porous Aramid Films Using Porosity Promoter Polymers. J. Appl. Polym. Sci. 2022, 139, e53192. [Google Scholar] [CrossRef]
- Trigo-López, M.; Vallejos Calzada, S.; Reglero-Ruiz, J.A.; García García, F.C.; García Perez, J.M.; Ibeas Cortés, S.; Mendia Jalon, A.; Muñoz Santamaría, A. Modelos de Aramida, Su Procedimiento de Preparación y Su Utilización En La Funcionalizacion de Nanomateriales de Carbono; No. ES 2 844 585 B2; University of Burgos: Burgos, Spain, 2021. [Google Scholar]
- Trigo-López, M.; Vallejos, S.; Reglero Ruiz, J.A.; García-Gómez, A.; Seara-Martínez, M.; García, F.C.; García, J.M. High-Performance Nanoporous Aramid Films Reinforced with Functionalized Carbon Nanocharges Using Ionic Liquids. Polymer 2020, 202, 122629. [Google Scholar] [CrossRef]
- Sousa, J.F.M.; Pina, J.; Gomes, C.; Dias, L.D.; Pereira, M.M.; Murtinho, D.; Dias, P.; Azevedo, J.; Mendes, A.; Seixas de Melo, J.S.; et al. Transport and Photophysical Studies on Porphyrin-Containing Sulfonated Poly(Etheretherketone) Composite Membranes. Mater. Today Commun. 2021, 29, 102781. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical Atomic Sensitivity Factors for Quantitative Analysis by Electron Spectroscopy for Chemical Analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Rubio-Aguinaga, A.; Reglero-Ruiz, J.A.; García-Gómez, A.; Peña Martín, E.; Ando, S.; Muñoz, A.; García, J.M.; Trigo-López, M. Boron Nitride-Reinforced Porous Aramid Composites with Enhanced Mechanical Performance and Thermal Conductivity. Compos. Sci. Technol. 2023, 242, 110211. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; de Villasante, A.G.; Asset, T.; Liu, Y.; Ly, A.; Pan, X.; Atanassov, P.; Zenyuk, I.V. Pyrolysis of Metal Organic Frameworks (MOF): Transformations Leading to Formation of Transition Metal-Nitrogen-Carbon Catalysts. ECS Meet. Abstr. 2021, MA2021-01, 476. [Google Scholar] [CrossRef]
- Chen, J.; Shen, B.; Jia, X.; Liu, Y.; Zheng, W. Lightweight and Compressible Anisotropic Honeycomb-like Graphene Composites for Highly Tunable Electromagnetic Shielding with Multiple Functions. Mater. Today Phys. 2022, 24, 100695. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Caoa, H.; Chena, Y.P. Raman Spectroscopy of Graphene and Related Materials. In New Developments in Photon and Materials Research; Jang, J.I., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 403–418. ISBN 9781626183391. [Google Scholar]
- Singh, K.; Baheti, V. Structure-Property Relationship of Carbonized Needlepunched Nonwovens for Electromagnetic Interference Shielding and Ohmic Heating Applications. Synth. Met. 2023, 292, 117220. [Google Scholar] [CrossRef]
- Villar-Rodil, S.; Suárez-García, F.; Paredes, J.I.; Martínez-Alonso, A.; Tascón, J.M.D. Activated Carbon Materials of Uniform Porosity from Polyaramid Fibers. Chem. Mater. 2005, 17, 5893–5908. [Google Scholar] [CrossRef]
- Castro-Muñiz, A.; Martínez-Alonso, A.; Tascón, J.M.D. Modification of the Pyrolysis/Carbonization of PPTA Polymer by Intermediate Isothermal Treatments. Carbon N. Y. 2008, 46, 985–993. [Google Scholar] [CrossRef]
- Kultayeva, S.; Ha, J.H.; Malik, R.; Kim, Y.W.; Kim, K.J. Effects of Porosity on Electrical and Thermal Conductivities of Porous SiC Ceramics. J. Eur. Ceram. Soc. 2020, 40, 996–1004. [Google Scholar] [CrossRef]
- Inagaki, M. Carbon Coating for Enhancing the Functionalities of Materials. Carbon N. Y. 2012, 50, 3247–3266. [Google Scholar] [CrossRef]
- Zhu, H.; Wei, J.; Wang, K.; Wu, D. Applications of Carbon Materials in Photovoltaic Solar Cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1461–1470. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.S.; Moradi, H.; Chang, Y.Y.; Yang, J.K. Highly Porous Biobased Graphene-like Carbon Adsorbent for Dye Removal: Preparation, Adsorption Mechanisms and Optimization. J. Environ. Chem. Eng. 2023, 11, 109278. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trigo-López, M.; Miguel, Á.; García, J.M.; Mendía, A.; Ruiz, V.; Valente, A.J.M.; Vallejos, S. Crafting and Analyzing Multi-Structured Aramid Materials and Their Pyrolytic Transformations: A Comprehensive Exploration. Polymers 2023, 15, 4315. https://doi.org/10.3390/polym15214315
Trigo-López M, Miguel Á, García JM, Mendía A, Ruiz V, Valente AJM, Vallejos S. Crafting and Analyzing Multi-Structured Aramid Materials and Their Pyrolytic Transformations: A Comprehensive Exploration. Polymers. 2023; 15(21):4315. https://doi.org/10.3390/polym15214315
Chicago/Turabian StyleTrigo-López, Miriam, Álvaro Miguel, José M. García, Aránzazu Mendía, Virginia Ruiz, Artur J. M. Valente, and Saúl Vallejos. 2023. "Crafting and Analyzing Multi-Structured Aramid Materials and Their Pyrolytic Transformations: A Comprehensive Exploration" Polymers 15, no. 21: 4315. https://doi.org/10.3390/polym15214315
APA StyleTrigo-López, M., Miguel, Á., García, J. M., Mendía, A., Ruiz, V., Valente, A. J. M., & Vallejos, S. (2023). Crafting and Analyzing Multi-Structured Aramid Materials and Their Pyrolytic Transformations: A Comprehensive Exploration. Polymers, 15(21), 4315. https://doi.org/10.3390/polym15214315