Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of CNCs
2.2.1. Preparation from Corn Stover
2.2.2. Color Measurement
2.2.3. Transmission Electron Microscopy (TEM)
2.3. Preparation and Characterization of Coating Solutions
2.3.1. Preparation
2.3.2. Droplet Size Analysis
2.3.3. Viscosity Assay
2.4. Preparation and Characterization of Coating Films
2.4.1. Preparation
2.4.2. Thickness Measurement of Coating Films
2.4.3. Optical Microscopy
2.4.4. UV-Vis
2.4.5. Tensile Testing
2.4.6. Water Vaper Permeability of Coating Films
2.5. Statistical Analysis
3. Results and Discussion
3.1. Yields and Color of CNCs Prepared from Corn Stover
3.2. Characterization of Corn Stover-Derived CNCs by Acid Hydrolysis
3.3. Characterization of Coating Solution
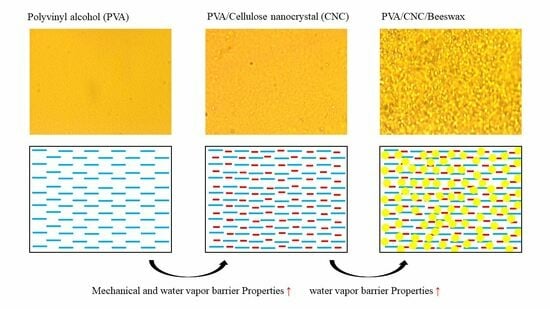
3.4. Characterization of Coating Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinstein, S.J.; Ruschak, K.J. Coating flows. Annu. Rev. Fluid Mech. 2004, 36, 29–53. [Google Scholar] [CrossRef]
- Gaur, P.K.; Mishra, S.; Gautam, R.; Singh, A.P.; Yasir, M. Film coating technology: Past, present and future. J. Pharm. Sci. Pharmacol. 2014, 1, 57–67. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-film coating methods: A successful marriage of high-quality and cost-effectiveness—A brief exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Sofyane, A.; Ben Ayed, E.; Lahcini, M.; Khouloud, M.; Kaddami, H.; Ameduri, B.; Boufi, S.; Raihane, M. Waterborne butyl methacrylate (co)polymers prepared by pickering emulsion polymerization: Insight of their use as coating materials for slow release-fertilizers. Eur. Polym. J. 2021, 156, 110598. [Google Scholar] [CrossRef]
- Fertahi, S.; Ilsouk, M.; Zeroual, Y.; Oukarroum, A.; Barakat, A. Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers. J. Control. Release 2021, 330, 341–361. [Google Scholar] [CrossRef]
- Ulaganathan, R.K.; Senusi, N.A.M.; Amin, M.A.M.; Razab, M.K.A.A.; Ismardi, A.; Abdullah, N.H. Effect of cellulose nanocrystals (CNC) on PVA/CNC bio-nanocomposite film as potential food packaging application. Mater. Today Proc. 2022, 66, 3150–3153. [Google Scholar] [CrossRef]
- Kassem, I.; Ablouh, E.-H.; El Bouchtaoui, F.-Z.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Ghalfi, H.; Alami, J.; El Achaby, M. Cellulose nanocrystals-filled poly (vinyl alcohol) nanocomposites as waterborne coating materials of NPK fertilizer with slow release and water retention properties. Int. J. Biol. Macromol. 2021, 189, 1029–1042. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for industrial use: Cellulose nanofibers (CNF), cellulose nanocrystals (CNC), and bacterial cellulose (BC). In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. [Google Scholar]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020, 331, 127108. [Google Scholar] [CrossRef] [PubMed]
- Jarosiewicz, A.; Tomaszewska, M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J. Agric. Food Chem. 2003, 51, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.M.; Smith, M.; Mekonnen, T.H. A nanomaterial-stabilized starch-beeswax Pickering emulsion coating to extend produce shelf-life. Chem. Eng. J. 2022, 431, 133905. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, H.; Qian, L. Beeswax–chitosan emulsion coated paper with enhanced water vapor barrier efficiency. Appl. Surf. Sci. 2014, 300, 80–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Simpson, B.K.; Dumont, M.-J. Effect of beeswax and carnauba wax addition on properties of gelatin films: A comparative study. Food Biosci. 2018, 26, 88–95. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Liu, L.; Gerard, G.; Peng, Z.; Yu, Z. The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion. Polymers 2023, 15, 757. [Google Scholar] [CrossRef]
- Costa, L.A.; Assis, D.D.J.; Gomes, G.V.; da Silva, J.B.; Fonsêca, A.F.; Druzian, J.I. Extraction and characterization of nanocellulose from corn stover. Mater. Today Proc. 2015, 2, 287–294. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Rajan, B.S.; Basha, K.S.; Madhu, P.; Raghav, G.R. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Costa, L.A.; Fonseca, A.F.; Pereira, F.V.; Druzian, J.I. Extraction and characterization of cellulose nanocrystals from corn stover. Cell. Chem. Technol. 2015, 49, 127–133. [Google Scholar]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, Y.; Wu, Q.; Wei, Z.; Lin, X.; Chen, Y. Preparation and characterization of cellulose nanocrystal extraction from pennisetum hydridum fertilized by municipal sewage sludge via sulfuric acid hydrolysis. Front. Energy Res. 2021, 9, 774783. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, E.; Kalic, P.; Kobol, A.; Ferreira, E.d.P. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason. Sonochem. 2016, 31, 473–480. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef]
- Kampeerapappun, P. Extraction and characterization of cellulose nanocrystals produced by acid hydrolysis from corn husk. J. Met. Mater. Miner. 2015, 25, 19–26. [Google Scholar]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Zhou, D. Cellulose nanocrystals (CNCs) from corn stalk: Activation energy analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef]
- Smyth, M.; García, A.; Rader, C.; Foster, E.; Bras, J. Extraction and process analysis of high aspect ratio cellulose nanocrystals from corn (Zea mays) agricultural residue. Ind. Crop. Prod. 2017, 108, 257–266. [Google Scholar] [CrossRef]
- Arakawa, C.K.; DeForest, C.A. Polymer design and development. In Biology and Engineering of Stem Cell Niches; Academic Press: Cambridge, MA, USA, 2017; pp. 295–314. [Google Scholar]
- Liu, K.; Liang, H.; Nasrallah, J.; Chen, L.; Huang, L.; Ni, Y. Preparation of the CNC/Ag/beeswax composites for enhancing antibacterial and water resistance properties of paper. Carbohydr. Polym. 2016, 142, 183–188. [Google Scholar] [CrossRef]
- Chu, W.-B.; Yang, J.-W.; Liu, T.-J.; Tiu, C.; Guo, J. The effects of pH, molecular weight and degree of hydrolysis of poly(vinyl alcohol) on slot die coating of PVA suspensions of TiO2 and SiO2. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 1–10. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Chen, L. Enhanced emulsifying properties of wood-based cellulose nanocrystals as Pickering emulsion stabilizer. Carbohydr. Polym. 2017, 169, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Musa, B.H.; Hameed, N.J. Study of the mechanical properties of polyvinyl alcohol/starch blends. Mater. Today Proc. 2020, 20, 439–442. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Liu, C.; Gan, L.; Ma, X.; Huang, J. Polydopamine-coated cellulose nanocrystals as an active ingredient in poly(vinyl alcohol) films towards intensifying packaging application potential. Cellulose 2019, 26, 9599–9612. [Google Scholar] [CrossRef]
- Oliveira, V.; Santos, F.; Leite, R.; Aroucha, E.; Silva, K. Use of biopolymeric coating hydrophobized with beeswax in post-harvest conservation of guavas. Food Chem. 2018, 259, 55–64. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ariahu, C.C.; Alakali, J.S. Effect of temperature and relative humidity on the water vapour permeability and mechanical properties of cassava starch and soy protein concentrate based edible films. J. Food Sci. Technol. 2015, 52, 2380–2386. [Google Scholar] [CrossRef]
- Krohn, J.; Tate, R.; Jordy, D. Factors affecting the permeability of PE blown films. J. Plast. Film Sheeting 1997, 13, 327–335. [Google Scholar] [CrossRef]
- Salazar, A.S.S.; Cavazos, P.A.S.; Paz, H.M.; Fragoso, A.V. External factors and nanoparticles effect on water vapor permeability of pectin-based films. J. Food Eng. 2019, 245, 73–79. [Google Scholar] [CrossRef]
- Othman, S.H.; Wane, B.M.; Nordin, N.; Hasnan, N.Z.N.; Talib, R.A.; Karyadi, J.N.W. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. [Google Scholar] [CrossRef]








| PVA * (g) | Glycerol (g) | Beeswax (g) | CNC (2%, w/w) (g) | DI Water (mL) | Final CNC Ratio (%, w/v) | |
|---|---|---|---|---|---|---|
| 1 | 3 | 0.6 | 0 | 0 | 100 | 0 |
| 2 | 3.8 | 96.2 | 0.075 | |||
| 3 | 7.5 | 92.5 | 0.15 | |||
| 4 | 15 | 85 | 0.3 | |||
| 5 | 1.5 | 3.8 | 96.2 | 0.075 | ||
| 6 | 7.5 | 92.5 | 0.15 | |||
| 7 | 15 | 85 | 0.3 |
| Raw | Washing | Alkaline Treatment | Bleaching | Acid Hydrolysis | |
|---|---|---|---|---|---|
| Step yield (%) | N/A | 74 ± 3 | 48 ± 1 | 69 ± 10 | 20 ± 5 |
| Overall yield (%) | N/A | 74 ± 3 | 35 ± 2 | 24 ± 4 | 5 ± 1 |
| L* | 30.4 ± 2.6 b | 32.7 ± 2.5 b | 31.8 ± 5.0 b | 43.2 ± 1.5 a | 34.4 ± 2.5 ab |
| a* | 3.6 ± 0.8 a | 3.9 ± 0.9 a | 2.9 ± 1.4 ab | 0.2 ± 0.1 c | 1.3 ± 0.0 b |
| b* | 13.1 ± 0.6 a | 12.9 ± 0.8 a | 11.6 ± 2.4 a | 3.1 ± 0.2 c | 5.3 ± 0.3 b |
| Samples | Composition | Film Thickness (mm) | ||
|---|---|---|---|---|
| Polyvinyl Alcohol (%, v/v) | Cellulose Nanocrystal (%, w/v) | Beeswax (%, w/v) | ||
| 1 | 3 | 0 | 0 | 0.10 ± 0.01 |
| 2 | 0.075 | 0.12 ± 0.01 | ||
| 3 | 0.15 | 0.13 ± 0.02 | ||
| 4 | 0.3 | 0.13 ± 0.02 | ||
| 5 | 0.075 | 1.5 | 0.13 ± 0.03 | |
| 6 | 0.15 | 0.14 ± 0.02 | ||
| 7 | 0.3 | 0.16 ± 0.02 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, N.; Friest, M.A.; Liu, L. Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax. Polymers 2023, 15, 4321. https://doi.org/10.3390/polym15214321
Park N, Friest MA, Liu L. Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax. Polymers. 2023; 15(21):4321. https://doi.org/10.3390/polym15214321
Chicago/Turabian StylePark, Namhyeon, Mason A. Friest, and Lingling Liu. 2023. "Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax" Polymers 15, no. 21: 4321. https://doi.org/10.3390/polym15214321
APA StylePark, N., Friest, M. A., & Liu, L. (2023). Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax. Polymers, 15(21), 4321. https://doi.org/10.3390/polym15214321