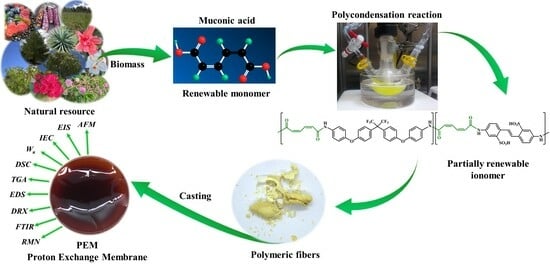
Synthesis, Characterization, and Proton Conductivity of Muconic Acid-Based Polyamides Bearing Sulfonated Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization Techniques
2.2. Reagents
2.3. Synthesis of the Polyamides
2.3.1. Characterization of Polymer MUFA
2.3.2. Characterization of Polymer MUFABA14
2.3.3. Characterization of Polymer MUFABA24
2.3.4. Characterization of Polymer MUFABA34
2.3.5. Characterization of Polymer MUFASA14
2.3.6. Characterization of Polymer MUFASA24
2.3.7. Characterization of Polymer MUFASA34
2.4. Membrane Preparation, Ion-Exchange Capacity, and Water Uptake
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, M.; Hong, S.-J.; Kim, N.-K.; Shin, J.; Kim, Y.-W. Vegetable Oil-Derived Polyamide Multiblock Copolymers toward Chemically Recyclable Pressure-Sensitive Adhesives. ACS Sustain. Chem. Eng. 2023, 11, 10095–10107. [Google Scholar] [CrossRef]
- Bottega Pergher, B.; Girigan, N.; Vlasblom, S.; Weinland, D.H.; Wang, B.; van Putten, R.-J.; Gruter, G.-J.M. Reactive Phenolic Solvents Applied to the Synthesis of Renewable Aromatic Polyesters with High Isosorbide Content. Polym. Chem. 2023, 14, 3225–3238. [Google Scholar] [CrossRef]
- Mu, T.; Leng, S.; Tang, W.; Shi, N.; Wang, G.; Yang, J. High-Performance and Low-Cost Membranes Based on Poly(Vinylpyrrolidone) and Cardo-Poly(Etherketone) Blends for Vanadium Redox Flow Battery Applications. Batteries 2022, 8, 230. [Google Scholar] [CrossRef]
- Mukoma, P.; Jooste, B.R.; Vosloo, H.C.M. Synthesis and Characterization of Cross-Linked Chitosan Membranes for Application as Alternative Proton Exchange Membrane Materials in Fuel Cells. J. Power Sources 2004, 136, 16–23. [Google Scholar] [CrossRef]
- Li, N.; Shin, D.W.; Hwang, D.S.; Lee, Y.M.; Guiver, M.D. Polymer Electrolyte Membranes Derived from New Sulfone Monomers with Pendent Sulfonic Acid Groups. Macromolecules 2010, 43, 9810–9820. [Google Scholar] [CrossRef]
- Li, J.; Pan, M.; Tang, H. Understanding Short-Side-Chain Perfluorinated Sulfonic Acid and Its Application for High Temperature Polymer Electrolyte Membrane Fuel Cells. RSC Adv. 2014, 4, 3944–3965. [Google Scholar] [CrossRef]
- Iulianelli, A.; Clarizia, G.; Gugliuzza, A.; Ebrasu, D.; Bevilacqua, A.; Trotta, F.; Basile, A. Sulfonation of PEEK-WC Polymer via Chloro-Sulfonic Acid for Potential PEM Fuel Cell Applications. Int. J. Hydrogen Energy 2010, 35, 12688–12695. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Zaidi, S.M.J.; Rahman, S.U. Proton Conductivity and Characterization of Novel Composite Membranes for Medium-Temperature Fuel Cells. Desalination 2006, 193, 387–397. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and Characterization of Sulfonated Poly(Ether Ether Ketone) for Proton Exchange Membranes. J. Memb. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Baç, N.; Eroǧlu, I. Preparation and Characterization of Sulfonated Polysulfone/Titanium Dioxide Composite Membranes for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Santiago, A.A.; Vargas, J.; Fomine, S.; Gaviño, R.; Tlenkopatchev, M.A. Polynorbornene with Pentafluorophenyl Imide Side Chain Groups: Synthesis and Sulfonation. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Dong, T.; Deng, Y.; Li, Y.; Lu, C.; Jia, W.; Meng, Z.; Zhou, M.; Tang, H. Phosphonate Poly(Vinylbenzyl Chloride)-Modified Sulfonated Poly(Aryl Ether Nitrile) for Blend Proton Exchange Membranes: Enhanced Mechanical and Electrochemical Properties. Polymers 2023, 15, 3203. [Google Scholar] [CrossRef]
- Zavorotnaya, U.M.; Ponomarev, I.I.; Volkova, Y.A.; Sinitsyn, V.V. Development of High-Performance Hydrogen-Air Fuel Cell with Flourine-Free Sulfonated Co-Polynaphthoyleneimide Membrane. Membranes 2023, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Poudel, M.B.; Chu, J.Y.; Vinothkannan, M.; Santhosh Kumar, R.; Logeshwaran, N.; Park, B.H.; Han, M.K.; Yoo, D.J. Advanced Performance and Ultra-High, Long-Term Durability of Acid-Base Blended Membranes over 900 Hours Containing Sulfonated PEEK and Quaternized Poly(Arylene Ether Sulfone) in H2/O2 Fuel Cells. Compos. Part B Eng. 2023, 254, 110558. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, M.; Huang, S.; Han, D.; Wang, S.; Meng, Y. Phosphonic Acid-Imidazolium Containing Polymer Ionomeric Membranes Derived from Poly (Phenylene Oxide) towards Boosting the Performance of HT-PEM Fuel Cells. J. Memb. Sci. 2023, 686, 121982. [Google Scholar] [CrossRef]
- Li, G.; Shen, R.; Hu, S.; Wang, B.; Algadi, H.; Wang, C. Norbornene-Based Acid–Base Blended Polymer Membranes with Low Ion Exchange Capacity for Proton Exchange Membrane Fuel Cell. Adv. Compos. Hybrid Mater. 2022, 5, 2131–2137. [Google Scholar] [CrossRef]
- Woroch, C.P.; Cox, I.W.; Kanan, M.W. A Semicrystalline Furanic Polyamide Made from Renewable Feedstocks. J. Am. Chem. Soc. 2023, 145, 697–705. [Google Scholar] [CrossRef]
- Reinaldo, J.S.; Milfont, C.H.R.; Gomes, F.P.C.; Mattos, A.L.A.; Medeiros, F.G.M.; Lopes, P.F.N.; Filho, M.d.S.M.S.; Matsui, K.N.; Ito, E.N. Influence of Grape and Acerola Residues on the Antioxidant, Physicochemical and Mechanical Properties of Cassava Starch Biocomposites. Polym. Test. 2021, 93, 107015. [Google Scholar] [CrossRef]
- Zarna, C.; Opedal, M.T.; Echtermeyer, A.T.; Chinga-Carrasco, G. Reinforcement Ability of Lignocellulosic Components in Biocomposites and Their 3D Printed Applications–A Review. Compos. Part C Open Access 2021, 6, 100171. [Google Scholar] [CrossRef]
- Terroba-Delicado, E.; Fiori, S.; Gomez-Caturla, J.; Montanes, N.; Sanchez-Nacher, L.; Torres-Giner, S. Valorization of Liquor Waste Derived Spent Coffee Grains for the Development of Injection-Molded Polylactide Pieces of Interest as Disposable Food Packaging and Serving Materials. Foods 2022, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Borrero-l, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications Of. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Wolff, S.; Rüppel, A.; Rida, H.A.; Heim, H.-P. Emission and Mechanical Properties of Glass and Cellulose Fiber Reinforced Bio-Polyamide Composites. Polymers 2023, 15, 2603. [Google Scholar] [CrossRef]
- Beppu, S.; Tachibana, Y.; Kasuya, K.I. Recyclable Polycarbosilane from a Biomass-Derived Bifuran-Based Monomer. ACS Macro Lett. 2023, 12, 536–542. [Google Scholar] [CrossRef]
- Khalil, I.; Quintens, G.; Junkers, T.; Dusselier, M. Muconic Acid Isomers as Platform Chemicals and Monomers in the Biobased Economy. Green Chem. 2020, 22, 1517–1541. [Google Scholar] [CrossRef]
- Quintens, G.; Vrijsen, J.H.; Adriaensens, P.; Vanderzande, D.; Junkers, T. Muconic Acid Esters as Bio-Based Acrylate Mimics. Polym. Chem. 2019, 10, 5555–5563. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Y.; Yuan, Y.; Zhang, T.; Su, T.; Li, Q.; Liang, Q.; Qi, Q. A Circular Bioprocess for the Sustainable Conversion of Polyethylene Terephthalate to Muconic Acid with an Engineered Pseudomonas Putida. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Molinari, F.; Pollegioni, L.; Rosini, E. Whole-Cell Bioconversion of Renewable Biomasses-Related Aromatics to Cis,Cis-Muconic Acid. ACS Sustain. Chem. Eng. 2023, 11, 2476–2485. [Google Scholar] [CrossRef]
- Nandhini, R.; Sivaprakash, B.; Rajamohan, N.; Vo, D.V.N. Lignin and Polylactic Acid for the Production of Bioplastics and Valuable Chemicals. Environ. Chem. Lett. 2022, 21, 403–427. [Google Scholar] [CrossRef]
- Weiland, F.; Barton, N.; Kohlstedt, M.; Becker, J.; Wittmann, C. Systems Metabolic Engineering Upgrades Corynebacterium Glutamicum to High-Efficiency Cis, Cis-Muconic Acid Production from Lignin-Based Aromatics. Metab. Eng. 2023, 75, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Maniar, D.; Fodor, C.; Karno Adi, I.; Woortman, A.J.J.; van Dijken, J.; Loos, K. Enzymatic Synthesis of Muconic Acid-Based Polymers: Trans, Trans-Dimethyl Muconate and Trans, β-Dimethyl Hydromuconate. Polymers 2021, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wang, J.; Wang, Z.; Yuan, L. Bio-Based Monomers for Amide-Containing Sustainable Polymers. Chem. Commun. 2023, 59, 382–400. [Google Scholar] [CrossRef]
- Santiago, A.A.; Ibarra-Palos, A.; Cruz-Morales, J.A.; Sierra, J.M.; Abatal, M.; Alfonso, I.; Vargas, J. Synthesis, Characterization, and Heavy Metal Adsorption Properties of Sulfonated Aromatic Polyamides. High Perform. Polym. 2018, 30, 591–601. [Google Scholar] [CrossRef]
- Ruiz, I.; Corona-García, C.; Santiago, A.A.; Abatal, M.; Téllez Arias, M.G.; Alfonso, I.; Vargas, J. Synthesis, Characterization, and Assessment of Novel Sulfonated Polynorbornene Dicarboximides as Adsorbents for the Removal of Heavy Metals from Water. Environ. Sci. Pollut. Res. 2021, 28, 52014–52031. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour-Ataei, S.; Hatami, M. Synthesis and Characterization of Novel Heat Resistant Poly(Amide Imide)S. Eur. Polym. J. 2005, 41, 2010–2015. [Google Scholar] [CrossRef]
- Pali-Casanova, R.d.J.; Yam-Cervantes, M.A.; Zavala-Loría, J.d.C.; Loría-Bastarrachea, M.I.; Aguilar-Vega, M.d.J.; Dzul-López, L.A.; Sámano-Celorio, M.L.; Crespo-álvarez, J.; García-Villena, E.; Agudo-Toyos, P.; et al. Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides. Polymers 2019, 11, 1169. [Google Scholar] [CrossRef]
- Ngadiwiyana; Gunawan; Prasetya, N.B.A.; Kusworo, T.D.; Susanto, H. Synthesis and Characterization of Sulfonated Poly(Eugenol-Co-Allyleugenol) Membranes for Proton Exchange Membrane Fuel Cells. Heliyon 2022, 8, e12401. [Google Scholar] [CrossRef]
- Pawlicka, A.; Mattos, R.I.; Tambelli, C.E.; Silva, I.D.A.; Magon, C.J.; Donoso, J.P. Magnetic Resonance Study of Chitosan Bio-Membranes with Proton Conductivity Properties. J. Memb. Sci. 2013, 429, 190–196. [Google Scholar] [CrossRef]
- Corona-García, C.; Onchi, A.; Santiago, A.A.; Martínez, A.; Pacheco-Catalán, D.E.; Alfonso, I.; Vargas, J. Synthesis and Characterization of Partially Renewable Oleic Acid-Based Ionomers for Proton Exchange Membranes. Polymers 2021, 13, 130. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Ochoa, N.A.; Takara, E.A.; Mollá, S.; Compañ, V. Influence of Pectin as a Green Polymer Electrolyte on the Transport Properties of Chitosan-Pectin Membranes. Carbohydr. Polym. 2017, 157, 1759–1768. [Google Scholar] [CrossRef]
- Farzin, S.; Johnson, T.J.; Chatterjee, S.; Zamani, E.; Dishari, S.K. Ionomers From Kraft Lignin for Renewable Energy Applications. Front. Chem. 2020, 8, 562278. [Google Scholar] [CrossRef]














| Polymer | MUA a (mmol) | HFDA b (mmol) | DABS c (mmol) | DASDA d (mmol) |
|---|---|---|---|---|
| MUFA | 0.64 | 0.64 | 0.00 | 0.00 |
| MUFABA14 | 0.64 | 0.48 | 0.16 | 0.00 |
| MUFABA24 | 0.64 | 0.32 | 0.32 | 0.00 |
| MUFABA34 | 0.64 | 0.16 | 0.48 | 0.00 |
| MUFASA14 | 0.64 | 0.48 | 0.00 | 0.16 |
| MUFASA24 | 0.64 | 0.32 | 0.00 | 0.32 |
| MUFASA34 | 0.64 | 0.16 | 0.00 | 0.48 |
| Polymer | Tg (°C) a | Td (°C) b | di (Å) c | ρ (g·cm−3) d | ηinh (dL·g−1) e |
|---|---|---|---|---|---|
| MUFA | 241 | 322 | 2.55 | 1.38 | 0.352 |
| MUFABA14 | 244 | 318 | 2.05 | 1.38 | 0.343 |
| MUFABA24 | 240 | 303 | 2.27 | 1.39 | 0.304 |
| MUFABA34 | 241 | 273 | 1.85 | 1.43 | 0.348 |
| MUFASA14 | 217 | 287 | 1.95 | 1.37 | 0.266 |
| MUFASA24 | 229 | 272 | 1.97 | 1.38 | 0.213 |
| MUFASA34 | 232 | 276 | 2.05 | 1.45 | 0.201 |
| Polymer | DS a (%) | IEC (mmol·g−1) | Wu (%) | (mS·cm−1) c | |
|---|---|---|---|---|---|
| Theoretical | Experimental b | ||||
| MUFA | - | - | - | 4.51 | - |
| MUFABA14 | 24.3 | 0.43 | 0.37 | 7.87 | 0.027 |
| MUFABA24 | 48.7 | 1.01 | 0.96 | 29.17 | 0.426 |
| MUFABA34 | 72.2 | 1.82 | 1.80 | 33.49 | 1.608 |
| MUFASA14 | 22.8 | 0.80 | 0.72 | 12.45 | 0.367 |
| MUFASA24 | 46.1 | 1.70 | 1.80 | 28.78 | 0.849 |
| MUFASA34 | 70.6 | 2.73 | 2.81 | 36.93 | 9.895 |
| Polymer | IECExp (meq·g−1) a | Wu (%) b | σp (mS·cm) c | Reference Author |
|---|---|---|---|---|
| (SPEAE) d | 0.356 | 51.2 | 0.018 | [36] |
| S5 e | - | - | 0.005 | [37] |
| DASA f | 2.2 | 44.1 | 0.5 | [38] |
| CH g | 0.37 | 249 | 2.44 | [39] |
| DAFASA2/4 f | 0.8 | 17.1 | 1.5 | [38] |
| LS 1.6 h | 1.6 | 25.9 | 8.12 | [40] |
| MUFABA34 | 1.80 | 33.49 | 1.608 | This study |
| MUFASA34 | 2.81 | 36.93 | 9.895 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-García, C.; Onchi, A.; Santiago, A.A.; Soto, T.E.; Vásquez-García, S.R.; Pacheco-Catalán, D.E.; Vargas, J. Synthesis, Characterization, and Proton Conductivity of Muconic Acid-Based Polyamides Bearing Sulfonated Moieties. Polymers 2023, 15, 4499. https://doi.org/10.3390/polym15234499
Corona-García C, Onchi A, Santiago AA, Soto TE, Vásquez-García SR, Pacheco-Catalán DE, Vargas J. Synthesis, Characterization, and Proton Conductivity of Muconic Acid-Based Polyamides Bearing Sulfonated Moieties. Polymers. 2023; 15(23):4499. https://doi.org/10.3390/polym15234499
Chicago/Turabian StyleCorona-García, Carlos, Alejandro Onchi, Arlette A. Santiago, Tania E. Soto, Salomón Ramiro Vásquez-García, Daniella Esperanza Pacheco-Catalán, and Joel Vargas. 2023. "Synthesis, Characterization, and Proton Conductivity of Muconic Acid-Based Polyamides Bearing Sulfonated Moieties" Polymers 15, no. 23: 4499. https://doi.org/10.3390/polym15234499
APA StyleCorona-García, C., Onchi, A., Santiago, A. A., Soto, T. E., Vásquez-García, S. R., Pacheco-Catalán, D. E., & Vargas, J. (2023). Synthesis, Characterization, and Proton Conductivity of Muconic Acid-Based Polyamides Bearing Sulfonated Moieties. Polymers, 15(23), 4499. https://doi.org/10.3390/polym15234499