Poly(Butylene Succinate) Hybrid Multi-Walled Carbon Nanotube/Iron Oxide Nanocomposites: Electromagnetic Shielding and Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
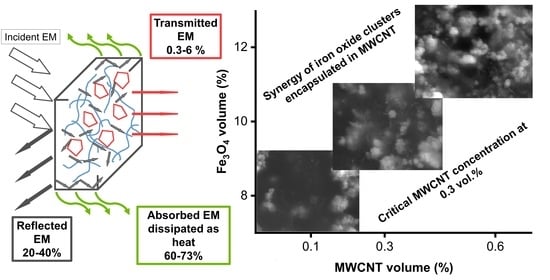
3. Results and Discussion
3.1. Structure
3.2. Light Flash Analysis
3.3. Activation Energy of Thermal Conductivity
3.4. Surface Resistivity Measurements and Response Surface Analysis
3.5. Broadband Dielectric Spectroscopy
3.6. High-Frequency Spectroscopy, Terahertz Transmittance, EMI Shielding Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.W.; Soren, K.; Dey, B.; Khan, M.S.; Choudhury, A. Synergistic reinforcement effect of 3D graphene@multi-walled carbon nanotube hybrid nanofiller in enhancing the electrical, EMI-shielding, and mechanical properties of polyethersulfone. Int. J. Polym. Anal. Charact. 2021, 26, 754–771. [Google Scholar] [CrossRef]
- Zeranska, K.; Filak, K.; Wilczyński, K.; Siemion, A.; Palka, N.; Godziszewski, K.; Yashchyshyn, Y.; Zdrojek, M. Graphene-Based Thermoplastic Composites as Extremely Broadband and Frequency-Dependent EMI Absorbers for Multifunctional Applications. ACS Appl. Electron. Mater. 2022, 4, 4463–4470. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Shadlou, S.; Shokrieh, M.M.; Chitsazzadeh, M. Effect of multi-walled carbon nanotube aspect ratio on mechanical and electrical properties of epoxy-based nanocomposites. Polym. Test. 2011, 30, 548–556. [Google Scholar] [CrossRef]
- Ohki, Y.; Hirai, N. Electrical conduction and breakdown properties of several biodegradable polymers. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1559–1566. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, L.; Yang, S. Polymer composites with expanded graphite network with superior thermal conductivity and electromagnetic interference shielding performance. Chem. Eng. J. 2021, 404, 126437. [Google Scholar] [CrossRef]
- Ferreira da Silva, T.; Menezes, F.; Montagna, L.S.; Lemes, A.P.; Passador, F.R. Synergistic effect of adding lignin and carbon black in poly(lactic acid). Polímeros 2020, 30, e2020002. [Google Scholar] [CrossRef]
- Bindu Sharmila, T.K.; Antony, J.V.; Jayakrishnan, M.P.; Sabura Beegum, P.M.; Thachil, E.T. Mechanical, thermal and dielectric properties of hybrid composites of epoxy and reduced graphene oxide/iron oxide. Mater. Des. 2016, 90, 66–75. [Google Scholar] [CrossRef]
- Li, K.; Fina, A.; Marrè, D.; Carosio, F.; Monticelli, O. Graphite oxide nanocoatings as a sustaibale route to extend the applicability of biopolymer-based film. Appl. Surf. Sci. 2020, 522, 146471. [Google Scholar] [CrossRef]
- Wei, Z.; Cai, C.; Huang, Y.; Wang, P.; Song, J.; Deng, L.; Fu, Y. Eco-friendly strategy to a dual-2D graphene-derived complex for poly(lactic acid) with exceptional smoke suppression and low CO2 production. J. Cleaner Prod. 2021, 280, 124433. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Z.; Smith, L.M.; Hong, G.; Song, W.; Zhang, S. Polypyrrole-modified bamboo fiber/polylactic acid with enhanced mechanical, the antistatic properties and thermal stability. Ind. Crops Prod. 2021, 162, 113227. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lee, C.-H.; Rajesh, R. Iron oxide-entrapped solid lipid nanoparticles and poly(lactide-co-glycolide) nanoparticles with surfactant stabilization for antistatic application. J. Mater. Res. Technol. 2019, 8, 887–895. [Google Scholar] [CrossRef]
- Tirado-Garcia, I.; Garcia-Gonzalez, D.; Garzon-Hernandez, S.; Rusinek, A.; Robles, G.; Martinez-Tarifa, J.M.; Arias, A. Conductive 3D printed PLA composites: On the interplay of mechanical, electrical and thermal behaviours. Compos. Struct. 2021, 265, 113744. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Du, Z.; Chen, K.; Zhang, Y.; Wang, Y.; He, P.; Mi, H.-Y.; Wang, Y.; Liu, C.; Shen, C. Engineering multilayered MXene/electrospun poly(lactic acid) membrane with increscent electromagnetic interference (EMI) shielding for integrated Joule heating and energy generating. Compos. Commun. 2021, 26, 100770. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Tian, G.; Wang, Y.; Gao, J.; Wang, C.; Xu, L.; Zhang, H. Morphology evolution to form double percolation polylactide/polycaprolactone/MWCNTs nanocomposites with ultralow percolation threshold and excellent EMI shielding. Compos. Sci. Technol. 2021, 214, 108956. [Google Scholar] [CrossRef]
- Lin, C.-S.; Shih, Y.-F.; Jeng, R.-J.; Dai, S.A.; Lin, J.-J.; Lee, C.-C. Nanocomposites with enhanced electrical properties based on biodegradable poly(butylene succinate) and polyetheramine modified carbon nanotube. J. Taiwan Inst. Chem. Eng. 2012, 43, 322–328. [Google Scholar] [CrossRef]
- Shi, Y.; He, L.; Chen, D.; Wang, Q.; Shen, J.; Guo, S. Simultaneously improved electromagnetic interference shielding and flame retarding properties of poly(butylene succinate)/thermoplastic polyurethane blends by constructing segregated flame retardants and multi-walled carbon nanotubes double network. Compos. Part A 2020, 137, 106037. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Wang, Q.; Chen, D.; Shen, J.; Guo, S. Strategy for constructing electromagnetic interference shielding and flame retarding synergistic network in poly(butylene succinate) and thermoplastic polyurethane multilayered composites. Compos. Sci. Technol. 2020, 199, 108324. [Google Scholar] [CrossRef]
- Luo, J.; Yin, D.; Yu, K.; Zhou, H.; Wen, B.; Wang, X. Facile Fabrication of PBS/CNTs Nanocomposite Foam for Electromagnetic Interference Shielding. Chemphyschem 2022, 23, e202100778. [Google Scholar] [CrossRef]
- Rincón-Iglesias, M.; Salado, M.; Lanceros-Mendez, S.; Lizundia, E. Magnetically active nanocomposites based on biodegradable polylactide, polycaprolactone, polybutylene succinate and polybutylene adipate terephthalate. Polymer 2022, 249, 124804. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4U, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Grigas, J. Microwave Dielectric Spectroscopy of Ferroelectrics. Ferroelectrics 2009, 380, 113–121. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevič, J.; Kuzhir, P.; Sokal, A.; Lapko, K.; Selskis, A.; Banys, J. Synergy Effects in Electromagnetic Properties of Phosphate Ceramics with Silicon Carbide Whiskers and Carbon Nanotubes. Appl. Sci. 2019, 9, 4388. [Google Scholar] [CrossRef] [Green Version]
- Plyushch, A.; Macutkevič, J.; Svirskas, S.; Banys, J.; Plausinaitiene, V.; Bychanok, D.; Maksimenko, S.A.; Selskis, A.; Sokal, A.; Lapko, K.N.; et al. Silicon carbide/phosphate ceramics composite for electromagnetic shielding applications: Whiskers vs particles. Appl. Phys. Lett. 2019, 114, 183105. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D. Magnetic Assembly of Superparamagnetic Iron Oxide Nanoparticle Clusters into Nanochains and Nanobundles. ACS Nano 2015, 9, 9700–9707. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Socoliuc, V.; Vékás, L. Hydrophobic and Hydrophilic Magnetite Nanoparticles: Synthesis by Chemical Coprecipitation and Physico-Chemical Characterization. In Upscaling of Bio-Nano-Processes; Nirschl, H., Keller, K., Eds.; Lecture Notes in Bioengineering; Springer: Heidelberg, Germany, 2014; pp. 39–55. ISBN 978-3-662-43898-5. [Google Scholar]
- Lattuada, M. Effect of Clustering on the Heat Generated by Superparamagnetic Iron Oxide Nanoparticles. Chimia 2019, 73, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.-Y.; Su, C.-H.; Yang, Y.-S.; Yeh, C.-S.; Tsai, C.-Y.; Wu, C.-L.; Wu, M.-T.; Shieh, D.-B. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 2005, 26, 729–738. [Google Scholar] [CrossRef]
- Kozakova, Z.; Kuritka, I.; Kazantseva, N.E.; Babayan, V.; Pastorek, M.; Machovsky, M.; Bazant, P.; Saha, P. The formation mechanism of iron oxide nanoparticles within the microwave-assisted solvothermal synthesis and its correlation with the structural and magnetic properties. Dalton Trans. 2015, 44, 21099–21108. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.; Mi, J.; Zhou, H.; Wang, X.; Tian, H. Fabrication of branching poly(butylene succinate)/cellulose nanocrystal foams with exceptional thermal insulation. Carbohydr. Polym. 2020, 247, 116708. [Google Scholar] [CrossRef]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R 2018, 132, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Gaidukovs, S.; Zukulis, E.; Bochkov, I.; Vaivodiss, R.; Gaidukova, G. Enhanced mechanical, conductivity, and dielectric characteristics of ethylene vinyl acetate copolymer composite filled with carbon nanotubes. J. Thermoplast. Compos. Mater. 2018, 31, 1161–1180. [Google Scholar] [CrossRef]
- Müller, A.J.; Arnal, M.L.; Trujillo, M.; Lorenzo, A.T. Super-nucleation in nanocomposites and confinement effects on the crystallizable components within block copolymers, miktoarm star copolymers and nanocomposites. Eur. Polym. J. 2011, 47, 614–629. [Google Scholar] [CrossRef] [Green Version]
- Habel, C.; Maiz, J.; Olmedo-Martínez, J.L.; López, J.V.; Breu, J.; Müller, A.J. Competition between nucleation and confinement in the crystallization of poly(ethylene glycol)/ large aspect ratio hectorite nanocomposites. Polymer 2020, 202, 122734. [Google Scholar] [CrossRef]
- Gohn, A.M.; Seo, J.; Colby, R.H.; Schaake, R.P.; Androsch, R.; Rhoades, A.M. Crystal nucleation in poly(ether ether ketone)/carbon nanotube nanocomposites at high and low supercooling of the melt. Polymer 2020, 199, 122548. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.S.; Kim, S.H. Unique nucleation of multi-walled carbon nanotube and poly(ethylene 2,6-naphthalate) nanocomposites during non-isothermal crystallization. Polymer 2006, 47, 1379–1389. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Shumakov, A.N.; Letenko, D.G.; Feldman, A.Y.; Marom, G. The nucleating effect of carbon nanotubes on crystallinity in R-BAPB-type thermoplastic polyimide. Macromol. Rapid Commun. 2005, 26, 885–888. [Google Scholar] [CrossRef]
- Yazdi, M.; Asl, V.H.; Pourmohammadi, M.; Roghani-Mamaqani, H. Mechanical properties, crystallinity, and self-nucleation of carbon nanotube-polyurethane nanocomposites. Polym. Test. 2019, 79, 106011. [Google Scholar] [CrossRef]
- Morcom, M.; Simon, G. Polyolefin-Carbon Nanotube Composites; McNally, T., Pötschke, P., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 511–544. ISBN 978-1-84569-761-7. [Google Scholar]
- Faridirad, F.; Barmar, M.; Ahmadi, S. The effect of MWCNT on dynamic mechanical properties and crystallinity of in situ polymerized polyamide 12 nanocomposite. Polym. Adv. Technol. 2018, 29, 2134–2146. [Google Scholar] [CrossRef]
- Chakoli, A.N.; Sui, J.; Amirian, M.; Cai, W. Crystallinity of biodegradable polymers reinforced with functionalized carbon nanotubes. J. Polym. Res. 2011, 18, 1249–1259. [Google Scholar] [CrossRef]
- Nunes Dos Santos, W.; Taylor, R. Effect of porosity on the thermal conductivity of alumina. High Temp.-High Press. 1993, 25, 89–98. [Google Scholar]
- dos Santos, W.N.; de Sousa, J.A.; Gregorio, R. Thermal conductivity behaviour of polymers around glass transition and crystalline melting temperatures. Polym. Test. 2013, 32, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Böer, K.W.; Pohl, U.W. Phonon-Induced Thermal Properties. In Semiconductor Physics; Böer, K.W., Pohl, U.W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 151–179. ISBN 978-3-319-06540-3. [Google Scholar]
- Cahill, D.G.; Pohl, R.O. Thermal conductivity of amorphous solids above the plateau. Phys. Rev. B 1987, 35, 4067–4073. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Waltermire, S.; Chen, Y.; Zinn, A.A.; Xu, T.T.; Li, D. Contact thermal resistance between individual multiwall carbon nanotubes. Appl. Phys. Lett. 2010, 96, 023109. [Google Scholar] [CrossRef]
- Pötschke, P.; Mothes, F.; Krause, B.; Voit, B. Melt-Mixed PP/MWCNT Composites: Influence of CNT Incorporation Strategy and Matrix Viscosity on Filler Dispersion and Electrical Resistivity. Polymers 2019, 11, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroni, L.G.; Soto-Oviedo, M.A.; Rosolen, J.M.; Felisberti, M.I.; Nogueira, A.F. Conductivity and mechanical properties of composites based on MWCNTs and styrene-butadiene-styrene block™ copolymers. J. Appl. Polym. Sci. 2009, 112, 3241–3248. [Google Scholar] [CrossRef]
- Jonscher, A.K. The universal dielectric response and its physical significance. IEEE Trans. Electr. Insul. 1992, 27, 407–423. [Google Scholar] [CrossRef]
- Meisak, D.; Macutkevic, J.; Plyushch, A.; Kuzhir, P.; Selskis, A.; Banys, J. Dielectric Relaxation in the Hybrid Epoxy/MWCNT/MnFe2O4 Composites. Polymers 2020, 12, 697. [Google Scholar] [CrossRef] [Green Version]
- Vanskevičė, I.; Kazakova, M.A.; Macutkevic, J.; Semikolenova, N.V.; Banys, J. Dielectric Properties of Hybrid Polyethylene Composites Containing Cobalt Nanoparticles and Carbon Nanotubes. Materials 2022, 15, 1876. [Google Scholar] [CrossRef]
- Essam, J.W. Percolation theory. Rep. Prog. Phys. 1980, 43, 833–912. [Google Scholar] [CrossRef]
- Bleija, M.; Platnieks, O.; Macutkevič, J.; Starkova, O.; Gaidukovs, S. Comparison of Carbon-Nanoparticle-Filled Poly(Butylene Succinate-co-Adipate) Nanocomposites for Electromagnetic Applications. Nanomaterials 2022, 12, 3671. [Google Scholar] [CrossRef]
- Marsden, A.J.; Papageorgiou, D.G.; Vallés, C.; Liscio, A.; Palermo, V.; Bissett, M.A.; Young, R.J.; Kinloch, I.A. Electrical percolation in graphene–polymer composites. 2D Mater. 2018, 5, 032003. [Google Scholar] [CrossRef] [Green Version]
- Platnieks, O.; Gaidukovs, S.; Neibolts, N.; Barkane, A.; Gaidukova, G.; Thakur, V.K. Poly(butylene succinate) and graphene nanoplatelet–based sustainable functional nanocomposite materials: Structure-properties relationship. Mater. Today Chem. 2020, 18, 100351. [Google Scholar] [CrossRef]
- Radoń, A.; Łukowiec, D.; Kremzer, M.; Mikuła, J.; Włodarczyk, P. Electrical Conduction Mechanism and Dielectric Properties of Spherical Shaped Fe3O4 Nanoparticles Synthesized by Co-Precipitation Method. Materials 2018, 11, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, F.; Brosseau, C. A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J. Appl. Phys. 2012, 111, 061301. [Google Scholar] [CrossRef]
- Sushmita, K.; Maiti, S.; Bose, S. Multi-layered composites using polyurethane-based foams and 3D-printed structures to curb electromagnetic pollution. Mater. Adv. 2022, 3, 4578–4599. [Google Scholar] [CrossRef]
- Tsonos, C.; Soin, N.; Tomara, G.; Yang, B.; Psarras, G.C.; Kanapitsas, A.; Siores, E. Electromagnetic wave absorption properties of ternary poly(vinylidene fluoride)/magnetite nanocomposites with carbon nanotubes and graphene. RSC Adv. 2016, 6, 1919–1924. [Google Scholar] [CrossRef]
- Polina, P.K.; Alesia, G.P.; Mikhail, V.S.; Sergey, A.M.; Alain, C.; Vanessa, F.; Gisele, A.-L.; Antonio, P.; Gintaras, V.; Jan, M.; et al. Electromagnetic shielding efficiency in Ka-band: Carbon foam versus epoxy/carbon nanotube composites. J. Nanophotonics 2012, 6, 061715. [Google Scholar] [CrossRef]










| No. | Sample | MWCNT (vol.%) | MWCNT (wt.%) | Fe3O4 (vol.%) | Fe3O4 (wt.%) | Surfactant (vol.%) | Surfactant (wt.%) |
|---|---|---|---|---|---|---|---|
| 1 | Ref | 0.00 | 0.00 | 0.00 | 0.00 | 0.51 | 0.45 |
| 2 | 01-8 | 0.10 | 0.12 | 8.20 | 26.55 | 0.50 | 0.35 |
| 3 | 01-10 | 0.10 | 0.11 | 10.24 | 31.59 | 0.51 | 0.34 |
| 4 | 01-12 | 0.10 | 0.11 | 12.28 | 36.17 | 0.54 | 0.34 |
| 5 | 03-8 | 0.30 | 0.36 | 8.20 | 26.52 | 0.51 | 0.36 |
| 6 | 03-10 | 0.30 | 0.34 | 10.24 | 31.57 | 0.50 | 0.34 |
| 7 | 03-12 | 0.30 | 0.32 | 12.28 | 36.16 | 0.50 | 0.32 |
| 8 | 06-8 | 0.60 | 0.72 | 8.20 | 26.49 | 0.50 | 0.35 |
| 9 | 06-10 | 0.60 | 0.69 | 10.24 | 31.52 | 0.53 | 0.35 |
| 10 | 06-12 | 0.60 | 0.65 | 12.28 | 36.11 | 0.50 | 0.32 |
| Sample | dEX (g/cm3) | dTH (g/cm3) * | Vp (%) |
|---|---|---|---|
| Ref | 1.264 ± 0.0011 | 1.259 | – |
| 01-8 | 1.566 ± 0.0059 | 1.575 | 0.57 |
| 01-10 | 1.618 ± 0.0082 | 1.653 | 2.12 |
| 01-12 | 1.717 ± 0.0064 | 1.731 | 0.81 |
| 03-8 | 1.562 ± 0.0059 | 1.576 | 0.89 |
| 03-10 | 1.649 ± 0.0057 | 1.654 | 0.30 |
| 03-12 | 1.708 ± 0.0068 | 1.733 | 1.44 |
| 06-8 | 1.564 ± 0.0068 | 1.578 | 0.89 |
| 06-10 | 1.652 ± 0.0034 | 1.656 | 0.24 |
| 06-12 | 1.724 ± 0.0062 | 1.734 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleija, M.; Platnieks, O.; Macutkevič, J.; Banys, J.; Starkova, O.; Grase, L.; Gaidukovs, S. Poly(Butylene Succinate) Hybrid Multi-Walled Carbon Nanotube/Iron Oxide Nanocomposites: Electromagnetic Shielding and Thermal Properties. Polymers 2023, 15, 515. https://doi.org/10.3390/polym15030515
Bleija M, Platnieks O, Macutkevič J, Banys J, Starkova O, Grase L, Gaidukovs S. Poly(Butylene Succinate) Hybrid Multi-Walled Carbon Nanotube/Iron Oxide Nanocomposites: Electromagnetic Shielding and Thermal Properties. Polymers. 2023; 15(3):515. https://doi.org/10.3390/polym15030515
Chicago/Turabian StyleBleija, Miks, Oskars Platnieks, Jan Macutkevič, Jūras Banys, Olesja Starkova, Liga Grase, and Sergejs Gaidukovs. 2023. "Poly(Butylene Succinate) Hybrid Multi-Walled Carbon Nanotube/Iron Oxide Nanocomposites: Electromagnetic Shielding and Thermal Properties" Polymers 15, no. 3: 515. https://doi.org/10.3390/polym15030515
APA StyleBleija, M., Platnieks, O., Macutkevič, J., Banys, J., Starkova, O., Grase, L., & Gaidukovs, S. (2023). Poly(Butylene Succinate) Hybrid Multi-Walled Carbon Nanotube/Iron Oxide Nanocomposites: Electromagnetic Shielding and Thermal Properties. Polymers, 15(3), 515. https://doi.org/10.3390/polym15030515