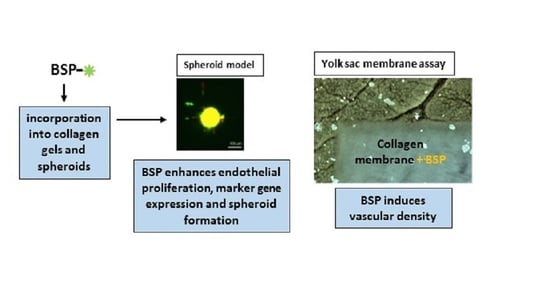
Bone Sialoprotein Immobilized in Collagen Type I Enhances Angiogenesis In Vitro and In Ovo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Collagen Gels (Modified after the Protocol from Wenger [37])
2.3. BSP-Release Assay
2.4. Viability Assay
2.5. RNA-Isolation/Reverse Transcription/Quantitative Real-Time PCR
2.6. Spheroid Model
2.6.1. Spheroid Preparation
2.6.2. Angiogenesis Assay
2.7. In Ovo—Yolk Sac Membrane Assay
2.8. Statistics
3. Results and Discussion
3.1. BSP Immobilized in Collagen Type I Enhances Proliferation of HUVECs
3.2. Effect of BSP Encapsulation in Collagen Gels on Gene Expression in Endothelial Cells
3.3. BSP Induces Sprout Number and Length in a Spheroid Model
3.4. BSP Enhances Vascular Density in the YSM-Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Walter, N.; Stich, T.; Docheva, D.; Alt, V.; Rupp, M. Evolution of implants and advancements for osseointegration: A narrative review. Injury 2022, 53 (Suppl. S3), S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Klute, L.; Baertl, S.; Walter, N.; Mannala, G.K.; Frank, L.; Pfeifer, C.; Alt, V.; Kerschbaum, M. The clinical use of bone graft substitutes in orthopedic surgery in Germany-A 10-years survey from 2008 to 2018 of 1,090,167 surgical interventions. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Papanagiotou, M.; Dailiana, Z.H.; Karachalios, T.; Varitimidis, S.; Hantes, M.; Dimakopoulos, G.; Vlychou, M.; Malizos, K.N. Heterotopic ossification after the use of recombinant human bone morphogenetic protein-7. World J. Orthop. 2017, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Vincent, K.; Durrant, M.C. A structural and functional model for human bone sialoprotein. J. Mol. Graph. Model. 2013, 39, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Mkhonto, D.; Cui, Q.; Sahai, N. Theoretical study of bone sialoprotein in bone biomineralization. Cells Tissues Organs 2011, 194, 182–187. [Google Scholar] [CrossRef]
- Bouleftour, W.; Juignet, L.; Bouet, G.; Granito, R.N.; Vanden-Bossche, A.; Laroche, N.; Aubin, J.E.; Lafage-Proust, M.H.; Vico, L.; Malaval, L. The role of the SIBLING, Bone Sialoprotein in skeletal biology—Contribution of mouse experimental genetics. Matrix. Biol. 2016, 52–54, 60–77. [Google Scholar] [CrossRef]
- Monfoulet, L.; Malaval, L.; Aubin, J.E.; Rittling, S.R.; Gadeau, A.P.; Fricain, J.C.; Chassande, O. Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during cortical defect healing. Bone 2010, 46, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Graf, H.L.; Stoeva, S.; Armbruster, F.P.; Neuhaus, J.; Hilbig, H. Effect of bone sialoprotein and collagen coating on cell attachment to TICER and pure titanium implant surfaces. Int. J. Oral. Maxillofac. Surg. 2008, 37, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, H.; Kirsten, M.; Rupietta, R.; Graf, H.L.; Thalhammer, S.; Strasser, S.; Armbruster, F.P. Implant surface coatings with bone sialoprotein, collagen, and fibronectin and their effects on cells derived from human maxillar bone. Eur. J. Med. Res. 2007, 12, 6–12. [Google Scholar] [PubMed]
- Baranowski, A.; Klein, A.; Ritz, U.; Ackermann, A.; Anthonissen, J.; Kaufmann, K.B.; Brendel, C.; Gotz, H.; Rommens, P.M.; Hofmann, A. Surface Functionalization of Orthopedic Titanium Implants with Bone Sialoprotein. PLoS ONE 2016, 11, e0153978. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.; Baranowski, A.; Ritz, U.; Gotz, H.; Heinemann, S.; Mattyasovszky, S.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coated three-dimensional printed calcium phosphate scaffolds on primary human osteoblasts. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Gallego-Llamas, J.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. In vivo biological responses to silk proteins functionalized with bone sialoprotein. Macromol. Biosci. 2013, 13, 444–454. [Google Scholar] [CrossRef]
- Baranowski, A.; Klein, A.; Ritz, U.; Gotz, H.; Mattyasovszky, S.G.; Rommens, P.M.; Hofmann, A. Evaluation of Bone Sialoprotein Coating of Three-Dimensional Printed Calcium Phosphate Scaffolds in a Calvarial Defect Model in Mice. Materials 2018, 11, 2336. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.; Baranowski, A.; Ritz, U.; Mack, C.; Gotz, H.; Langendorf, E.; Al-Nawas, B.; Drees, P.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coating on progression of bone formation in a femoral defect model in rats. Eur. J. Trauma. Emerg. Surg. 2020, 46, 277–286. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Maniglia, B.C.; Buchet, R.; Millan, J.L.; Ciancaglini, P.; Bottini, M.; Ramos, A.P. Three-dimensional cell-laden collagen scaffolds: From biochemistry to bone bioengineering. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 967–983. [Google Scholar] [CrossRef]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem. Cells 2015, 7, 657–668. [Google Scholar] [CrossRef]
- Tye, C.E.; Hunter, G.K.; Goldberg, H.A. Identification of the type I collagen-binding domain of bone sialoprotein and characterization of the mechanism of interaction. J. Biol. Chem. 2005, 280, 13487–13492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorski, J.P.; Franz, N.T.; Pernoud, D.; Keightley, A.; Eyre, D.R.; Oxford, J.T. A repeated triple lysine motif anchors complexes containing bone sialoprotein and the type XI collagen A1 chain involved in bone mineralization. J. Biol. Chem. 2021, 296, 100436. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.; Schlosser, C.; Habeck, T.; Dahmen, C.; Gotz, H.; Clauder, F.; Armbruster, F.P.; Baranowski, A.; Drees, P.; Rommens, P.M.; et al. Bone Sialoprotein Immobilized in Collagen Type I Enhances Bone Regeneration In vitro and In vivo. Int. J. Bioprint. 2022, 8, 591. [Google Scholar] [CrossRef]
- Mahapatra, C.; Kumar, P.; Paul, M.K.; Kumar, A. Angiogenic stimulation strategies in bone tissue regeneration. Tissue Cell. 2022, 79, 101908. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Jhala, D.; Vasita, R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater Sci. Eng. C Mater Biol. Appl. 2019, 103, 109761. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, A.; Bonjean, K.; Fohr, B.; Fedarko, N.S.; Robey, F.A.; Young, M.F.; Fisher, L.W.; Castronovo, V. Bone sialoprotein mediates human endothelial cell attachment and migration and promotes angiogenesis. Circ. Res. 2000, 86, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byzova, T.V.; Kim, W.; Midura, R.J.; Plow, E.F. Activation of integrin alpha(V)beta(3) regulates cell adhesion and migration to bone sialoprotein. Exp. Cell. Res. 2000, 254, 299–308. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Mashimo, Y.; Mie, M.; Kobatake, E. Temperature-Responsive Multifunctional Protein Hydrogels with Elastin-like Polypeptides for 3-D Angiogenesis. Biomacromolecules 2020, 21, 1126–1135. [Google Scholar] [CrossRef]
- Jain, A.; Fisher, L.W.; Fedarko, N.S. Bone sialoprotein binding to matrix metalloproteinase-2 alters enzyme inhibition kinetics. Biochemistry 2008, 47, 5986–5995. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Karadag, A.; Fisher, L.W.; Fedarko, N.S. Structural requirements for bone sialoprotein binding and modulation of matrix metalloproteinase-2. Biochemistry 2008, 47, 10162–10170. [Google Scholar] [CrossRef] [Green Version]
- Korff, T.; Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell. Biol. 1998, 143, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Heiss, M.; Hellstrom, M.; Kalen, M.; May, T.; Weber, H.; Hecker, M.; Augustin, H.G.; Korff, T. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 2015, 29, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, L.; Korff, T. Spheroid-Based In Vitro Angiogenesis Model. Methods Mol. Biol. 2016, 1430, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Lu, C.Y.; Wang, X.H.; Bao, Y.L.; Meng, X.Y.; Wu, Y.; Li, Y.X. Chick yolk sac membrane assay: A novel angiogenesis model. J. Biol. Res. Thessalon 2007, 7, 93–97. [Google Scholar]
- As, M.N.; Deshpande, R.; Kale, V.P.; Bhonde, R.R.; Datar, S.P. Establishment of an in ovo chick embryo yolk sac membrane (YSM) assay for pilot screening of potential angiogenic and anti-angiogenic agents. Cell. Biol. Int. 2018, 42, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Ritz, U.; Verrier, S.; Eglin, D.; Alini, M.; Fuchs, S.; Kirkpatrick, C.J.; Rommens, P.M. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 2008, 29, 4217–4226. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.; Stahl, A.; Weber, H.; Finkenzeller, G.; Augustin, H.G.; Stark, G.B.; Kneser, U. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004, 10, 1536–1547. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Eglinger, J.; Karsjens, H.; Lammert, E. Quantitative assessment of angiogenesis and pericyte coverage in human cell-derived vascular sprouts. Inflamm. Regen. 2017, 37, 2. [Google Scholar] [CrossRef] [Green Version]
- Arrabal, P.M.; Visser, R.; Santos-Ruiz, L.; Becerra, J.; Cifuentes, M. Osteogenic molecules for clinical applications: Improving the BMP-collagen system. Biol. Res. 2013, 46, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Bierbaum, S.; Hintze, V.; Scharnweber, D. Functionalization of biomaterial surfaces using artificial extracellular matrices. Biomatter 2012, 2, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Anderson, A.L.; Lu, Q.; Wang, J. Role of fibrillar structure of collagenous carrier in bone sialoprotein-mediated matrix mineralization and osteoblast differentiation. Biomaterials 2007, 28, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix. Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Q.; Shao, X.; Zhang, T.; Xue, C.; Shi, S.; Zhao, D.; Lin, Y. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell. Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local delivery of insulin/IGF-1 for bone regeneration: Carriers, strategies, and effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Bouleftour, W.; Boudiffa, M.; Wade-Gueye, N.M.; Bouet, G.; Cardelli, M.; Laroche, N.; Vanden-Bossche, A.; Thomas, M.; Bonnelye, E.; Aubin, J.E.; et al. Skeletal development of mice lacking bone sialoprotein (BSP)--impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS ONE 2014, 9, e95144. [Google Scholar] [CrossRef]
- Lu, W.; Xu, W.; Li, J.; Chen, Y.; Pan, Y.; Wu, B. Effects of vascular endothelial growth factor and insulin growth factor1 on proliferation, migration, osteogenesis and vascularization of human carious dental pulp stem cells. Mol. Med. Rep. 2019, 20, 3924–3932. [Google Scholar] [CrossRef]
- Nakayama, Y.; Nakajima, Y.; Kato, N.; Takai, H.; Kim, D.S.; Arai, M.; Mezawa, M.; Araki, S.; Sodek, J.; Ogata, Y. Insulin-like growth factor-I increases bone sialoprotein (BSP) expression through fibroblast growth factor-2 response element and homeodomain protein-binding site in the proximal promoter of the BSP gene. J. Cell. Physiol. 2006, 208, 326–335. [Google Scholar] [CrossRef]
- Rustamov, V.; Keller, F.; Klicks, J.; Hafner, M.; Rudolf, R. Bone Sialoprotein Shows Enhanced Expression in Early, High-Proliferation Stages of Three-Dimensional Spheroid Cell Cultures of Breast Cancer Cell Line MDA-MB-231. Front. Oncol. 2019, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Unger, R.E.; Dohle, E.; Kirkpatrick, C.J. Improving vascularization of engineered bone through the generation of pro-angiogenic effects in co-culture systems. Adv. Drug Deliv. Rev. 2015, 94, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Shahabipour, F.; Tavafoghi, M.; Aninwene, G.E., 2nd; Bonakdar, S.; Oskuee, R.K.; Shokrgozar, M.A.; Potyondy, T.; Alambeigi, F.; Ahadian, S. Coaxial 3D bioprinting of tri-polymer scaffolds to improve the osteogenic and vasculogenic potential of cells in co-culture models. J. Biomed. Mater. Res. A 2022, 110, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Kocherova, I.; Bryja, A.; Mozdziak, P.; Angelova Volponi, A.; Dyszkiewicz-Konwinska, M.; Piotrowska-Kempisty, H.; Antosik, P.; Bukowska, D.; Bruska, M.; Izycki, D.; et al. Human Umbilical Vein Endothelial Cells (HUVECs) Co-Culture with Osteogenic Cells: From Molecular Communication to Engineering Prevascularised Bone Grafts. J. Clin. Med. 2019, 8, 1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, B.; Bastos, A.R.F.; Brancato, V.; Cerqueira, M.T.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Mechanical Property of Hydrogels and the Presence of Adipose Stem Cells in Tumor Stroma Affect Spheroid Formation in the 3D Osteosarcoma Model. ACS Appl. Mater Interfaces 2019, 11, 14548–14559. [Google Scholar] [CrossRef] [PubMed]
- Im, B.J.; Lee, S.C.; Lee, M.H.; Leesungbok, R.; Ahn, S.J.; Kang, Y.G.; Lee do, Y.; Yoon, J.H.; Lee, S.W. Promotion of osteoblastic differentiation and osteogenic transcription factor expression on a microgroove titanium surface with immobilized fibronectin or bone sialoprotein II. Biomed. Mater 2016, 11, 035020. [Google Scholar] [CrossRef] [PubMed]
- Schaeren, S.; Jaquiery, C.; Wolf, F.; Papadimitropoulos, A.; Barbero, A.; Schultz-Thater, E.; Heberer, M.; Martin, I. Effect of bone sialoprotein coating of ceramic and synthetic polymer materials on in vitro osteogenic cell differentiation and in vivo bone formation. J. Biomed. Mater Res. A 2010, 92, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, N.; Banfi, A. The osteo-angiogenic signaling crosstalk for bone regeneration: Harmony out of complexity. Curr. Opin. Biotechnol. 2022, 76, 102750. [Google Scholar] [CrossRef]





| Gen | Forward Primer | Reverse Primer |
|---|---|---|
| b2-microglobulin | CTC ACG TCA TCC AGC AGA GA | ACG GCA GGC ATA CTC ATC TT |
| IGF-1 | CCT GAC CTT GTG ATT TGC CC | TCC CCT TGA AAG ACC CCA TC |
| KDR | TTA CTT GCA GGG GAC AGA GG | TTC CCG GTA GAA GCA CTT GT |
| MCAM | CGG CAA GTG AAC AAG ACC AA | GTC TGG TGT GAG GGT GGT TA |
| PECAM | CAT TGG CGT GTT GGG AAG AA | GCT CAT GTT TGC CTA GCT CC |
| VEGF | AGA TGA GCT TCC TAC AGC ACA AC | AGG ACT TAT ACC GGG ATT TCT TG |
| vWF | GGA TTC AGT GGA TGC AGC AG | TAG GGA GGT CTT CGA TTC GC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriegel, A.; Langendorf, E.; Kottmann, V.; Kämmerer, P.W.; Armbruster, F.P.; Wiesmann-Imilowski, N.; Baranowski, A.; Gercek, E.; Drees, P.; Rommens, P.M.; et al. Bone Sialoprotein Immobilized in Collagen Type I Enhances Angiogenesis In Vitro and In Ovo. Polymers 2023, 15, 1007. https://doi.org/10.3390/polym15041007
Kriegel A, Langendorf E, Kottmann V, Kämmerer PW, Armbruster FP, Wiesmann-Imilowski N, Baranowski A, Gercek E, Drees P, Rommens PM, et al. Bone Sialoprotein Immobilized in Collagen Type I Enhances Angiogenesis In Vitro and In Ovo. Polymers. 2023; 15(4):1007. https://doi.org/10.3390/polym15041007
Chicago/Turabian StyleKriegel, Anja, Eva Langendorf, Valentina Kottmann, Peer W. Kämmerer, Franz Paul Armbruster, Nadine Wiesmann-Imilowski, Andreas Baranowski, Erol Gercek, Philipp Drees, Pol Maria Rommens, and et al. 2023. "Bone Sialoprotein Immobilized in Collagen Type I Enhances Angiogenesis In Vitro and In Ovo" Polymers 15, no. 4: 1007. https://doi.org/10.3390/polym15041007
APA StyleKriegel, A., Langendorf, E., Kottmann, V., Kämmerer, P. W., Armbruster, F. P., Wiesmann-Imilowski, N., Baranowski, A., Gercek, E., Drees, P., Rommens, P. M., & Ritz, U. (2023). Bone Sialoprotein Immobilized in Collagen Type I Enhances Angiogenesis In Vitro and In Ovo. Polymers, 15(4), 1007. https://doi.org/10.3390/polym15041007