Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Method
2.2.1. Fabrication of AM-CS
2.2.2. Fabrication of Surface Functionalization of AM-CS
2.2.3. Particle Size, Polydispersity Index (PDI), and Zeta Potential
2.2.4. Morphology Studies
2.2.5. Determination of Entrapment Efficiency and Drug Loading
2.2.6. Fourier-Transform Infrared Spectroscopy Analysis
2.2.7. X-ray Diffraction Analysis
2.2.8. Differential Scanning Calorimetry Analysis
2.2.9. In Vitro Release Studies
2.2.10. Cytotoxicity Studies
2.2.11. Statistical Analysis
3. Results
3.1. Characterization of AM Nanoparticles
3.1.1. Particle Size, PDI, Zeta Potential, Morphology, EE, and DL
3.1.2. FTIR Analysis
3.1.3. XRD Analysis
3.1.4. DSC Analysis
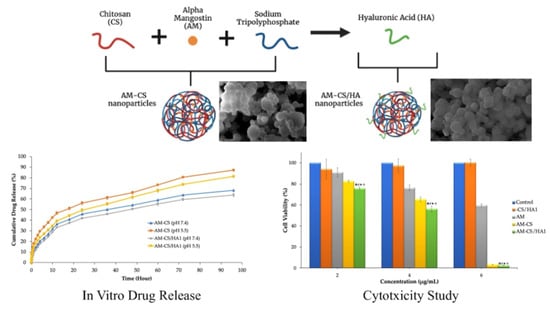
3.2. In Vitro Release Studies
3.3. Cytotoxicity Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Xu, B. Breast Cancer: An up-to-Date Review and Future Perspectives. Cancer Commun. 2022, 42, 913–936. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Dai, Y.; Gao, H. Development and Application of Hyaluronic Acid in Tumor Targeting Drug Delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Kritsanawong, S.; Innajak, S.; Imoto, M.; Watanapokasin, R. Antiproliferative and Apoptosis Induction of α-Mangostin in T47D Breast Cancer Cells. Int. J. Oncol. 2016, 48, 2155–2165. [Google Scholar] [CrossRef] [Green Version]
- Shibata, M.A.; Iinuma, M.; Morimoto, J.; Kurose, H.; Akamatsu, K.; Okuno, Y.; Akao, Y.; Otsuki, Y. α-Mangostin Extracted from the Pericarp of the Mangosteen (Garcinia mangostana Linn) Reduces Tumor Growth and Lymph Node Metastasis in an Immunocompetent Xenograft Model of Metastatic Mammary Cancer Carrying a P53 Mutation. BMC Med. 2011, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Benjakul, R.; Kongkaneramit, L.; Sarisuta, N.; Moongkarndi, P.; Müller-Goymann, C.C. Cytotoxic Effect and Mechanism Inducing Cell Death of α-Mangostin Liposomes in Various Human Carcinoma and Normal Cells. Anti-Cancer Drugs 2015, 26, 824–834. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Wijaya, C.A. Anticancer Potential of α-Mangostin. Asian J. Pharm. Clin. Res. 2017, 10, 440–445. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Kamalidehghan, B.; Ghaderian, M.; Dehghan, F.; Ali, L.Z.; Arbab, I.A.; Yahayu, M.; Lian, G.E.C. α-Mangostin from Cratoxylum Arborescens Demonstrates Apoptogenesis in MCF-7 with Regulation of NF-ΚB and Hsp70 Protein Modulation in Vitro, and Tumor Reduction in Vivo. Drug Des. Devel. Ther. 2014, 8, 1629–1647. [Google Scholar] [CrossRef] [Green Version]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-Mangostin for Cancer Drug Delivery System. Pharmaceutics 2021, 13, 1993. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Yu, W.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. α-Mangostin-Encapsulated PLGA Nanoparticles Inhibit Pancreatic Carcinogenesis by Targeting Cancer Stem Cells in Human, and Transgenic (KrasG12D, and KrasG12D/Tp53R270H) Mice. Sci. Rep. 2016, 6, 32743. [Google Scholar] [CrossRef]
- Gutierrez-Orozco, F.; Failla, M.L. Biological Activities and Bioavailability of Mangosteen Xanthones: A Critical Review of the Current Evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Brunner, I.; Han, A.R.; Hamburger, M.; Kinghorn, A.D.; Frye, R.; Butterweck, V. Pharmacokinetics of α-Mangostin in Rats after Intravenous and Oral Application. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), 67–74. [Google Scholar] [CrossRef]
- Aydin, R.S.T. Herceptin-Decorated Salinomycin-Loaded Nanoparticles for Breast Tumor Targeting. J. Biomed Mater. Res.—Part A 2013, 101, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric Nanoparticles for Targeted Drug Delivery System for Cancer Therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and Cancer Therapy: Perspectives for Application of Nanoparticles in the Treatment of Cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Riaz, M.; Yasmeen, E.; Yousaf, M. Recent Advances in Nanoparticle-Based Targeted Drug-Delivery Systems against Cancer and Role of Tumor Microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 317–353. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Perumal, S. Polymer Nanoparticles: Synthesis and Applications. Polymers 2022, 14, 5449. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Essiet Imeh, A.; Li, W. Polymeric Nanoparticles—Promising Carriers for Cancer Therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium Alginate-f-GO Composite Hydrogels for Tissue Regeneration and Antitumor Applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Al-Arjan, W.S.; Ashammakhi, N.; Haider, S.; Amin, R.; Hasan, A. Multifunctional Bioactive Scaffolds from ARX-g-(Zn@rGO)-HAp for Bone Tissue Engineering: In Vitro Antibacterial, Antitumor, and Biocompatibility Evaluations. ACS Appl. Bio Mater. 2022, 5, 5445–5456. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.; Laouini, A.; Fessi, H.; Charcosset, C. Preparation of Chitosan-TPP Nanoparticles Using Microengineered Membranes—Effect of Parameters and Encapsulation of Tacrine. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 34–43. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. PH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Wathoni, N.; Meylina, L.; Rusdin, A.; Abdelwahab Mohammed, A.F.; Tirtamie, D.; Herdiana, Y.; Motoyama, K.; Panatarani, C.; Joni, I.M.; Lesmana, R.; et al. The Potential Cytotoxic Activity Enhancement of α-Mangostin in Chitosan-Kappa Carrageenan-Loaded Nanoparticle against Mcf-7 Cell Line. Polymers 2021, 13, 1681. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Febriani, E.; Purnama, D.; Daulay, W.; Azhary, S.Y.; Panatarani, C.; Joni, I.M.; Lesmana, R.; Keiichi, M.; et al. Formulation and Characterization of α-Mangostin in Chitosan Nanoparticles Coated by Sodium Alginate, Sodium Silicate, and Polyethylene Glycol. J. Pharm. Bioallied Sci. 2019, 20, S619. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Tu, Y.; Yao, Z.; Yang, W.; Tao, S.; Li, B.; Wang, Y.; Su, Z.; Li, S. Application of Nanoparticles in Tumour Targeted Drug Delivery and Vaccine. Front. Nanotechnol. 2022, 4, 948705. [Google Scholar] [CrossRef]
- Kher, C.; Kumar, S. The Application of Nanotechnology and Nanomaterials in Cancer Diagnosis and Treatment: A Review. Cureus 2022, 14, e29059. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.A.; Kim, J.H.; Ryu, K.; Kaushik, N. Current Nanomedicine for Targeted Vascular Disease Treatment: Trends and Perspectives. Int. J. Mol. Sci. 2022, 23, 2397. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhai, J.; Hu, D.; Yang, R.; Wang, G.; Li, Y.; Liang, G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics 2022, 14, 1919. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Q.; Xia, X.; Li, X.; Ruan, W.; Zheng, M.; Zou, Y.; Shi, B. Polymeric Nanoparticles for Mitochondria Targeting Mediated Robust Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 755727. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Nikitin, P.I.; Rozenberg, J.M. Systematic Review of Cancer Targeting by Nanoparticles Revealed a Global Association between Accumulation in Tumors and Spleen. Int. J. Mol. Sci. 2021, 22, 13011. [Google Scholar] [CrossRef]
- Peltonen, L.; Singhal, M.; Hirvonen, J. Principles of Nanosized Drug Delivery Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as Carriers for Drug Delivery in Cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 295–305. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, F.; Peng, Y.; Xie, T.; Wang, Y.; Lan, Y. Current Progress in Cancer Treatment Using Nanomaterials. Front. Oncol. 2022, 12, 930125. [Google Scholar] [CrossRef]
- Zhou, Z.; Badkas, A.; Stevenson, M.; Lee, J.Y.; Leung, Y.K. Herceptin Conjugated PLGA-PHis-PEG PH Sensitive Nanoparticles for Targeted and Controlled Drug Delivery. Int. J. Pharm. 2015, 487, 81–90. [Google Scholar] [CrossRef]
- Jurj, A.; Braicu, C.; Pop, L.A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The New Era of Nanotechnology, an Alternative to Change Cancer Treatment. Drug Des. Devel. Ther. 2017, 11, 2871–2890. [Google Scholar] [CrossRef] [Green Version]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic Acid for Anticancer Drug and Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.H.; Geneidi, A.S.; Mansour, S. Surface Functionalization of Methotrexate-Loaded Chitosan Nanoparticles with Hyaluronic Acid/Human Serum Albumin: Comparative Characterization and in Vitro Cytotoxicity. Int. J. Pharm. 2017, 522, 128–136. [Google Scholar] [CrossRef]
- Louderbough, J.M.V.; Schroeder, J.A. Understanding the Dual Nature of CD44 in Breast Cancer Progression. Mol. Cancer Res. 2011, 9, 1573–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Othman, N.; Alhendi, A.; Ihbaisha, M.; Barahmeh, M.; Alqaraleh, M.; Al-Momany, B.Z. Role of CD44 in Breast Cancer. Breast Dis. 2020, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Koli, U.; Dandekar, P.; Jain, R. Investigating Behaviour of Polymers in Nanoparticles of Chitosan Oligosaccharides Coated with Hyaluronic Acid. Polymer 2016, 93, 44–52. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, H.J.; Yoon, H.Y.; Yoon, I.S.; Ko, S.H.; Shim, J.S.; Cho, J.H.; Park, J.H.; Kim, K.; Kwon, I.C.; et al. Hyaluronic Acid Derivative-Coated Nanohybrid Liposomes for Cancer Imaging and Drug Delivery. J. Control. Release 2014, 174, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Asati, P.; Jain, P.; Mishra, P.; Mishra, A.; Singour, P. Recent Advancements in Cancer Targeting Therapy with the Hyaluronic Acid as a Potential Adjuvant Avances Recientes En La Terapia Dirigida Al Cáncer Con El Ácido Hialurónico Como Adyuvante Potencial. Ars Pharm. 2022, 63, 387–409. [Google Scholar] [CrossRef]
- Elamin, K.M.; Yamashita, Y.; Higashi, T.; Motoyama, K.; Arima, H. Supramolecular Complex of Methyl-β-Cyclodextrin with Adamantane-Grafted Hyaluronic Acid as a Novel Antitumor Agent. Chem. Pharm. Bull. 2018, 66, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elamin, K.M.; Motoyama, K.; Higashi, T.; Yamashita, Y.; Tokuda, A.; Arima, H. Dual Targeting System by Supramolecular Complex of Folate-Conjugated Methyl-β-Cyclodextrin with Adamantane-Grafted Hyaluronic Acid for the Treatment of Colorectal Cancer. Int. J. Biol. Macromol. 2018, 113, 386–394. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Svechkrev, D.; Soucheck, J.; Hill, T.; Taylor, M.; Natarajan, A.; Mohs, A. Impact of Structurally Modifying Hyaluronic Acid on CD44 Interaction. J. Mater. Chem. B 2017, 5, 8183–8192. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, S.; Wang, C.; Sun, T.; Yang, L. Hyaluronic Acid-Based Nano Drug Delivery Systems for Breast Cancer Treatment: Recent Advances. Front. Bioeng. Biotechnol. 2022, 10, 990145. [Google Scholar] [CrossRef]
- Nasti, A.; Zaki, N.M.; De Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and Chitosan/TPP-Hyaluronic Acid Nanoparticles: Systematic Optimisation of the Preparative Process and Preliminary Biological Evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic Acid-Coated Chitosan Nanoparticles: Molecular Weight-Dependent Effects on Morphology and Hyaluronic Acid Presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic Acid-Coated Chitosan Nanoparticles as Targeted-Carrier of Tamoxifen against MCF7 and TMX-Resistant MCF7 Cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Abdulmajid, A.M.S.; Ismail, Z.; Alrokayan, S.A.; Abu-Salah, K.M. Development of Polymeric Nanoparticles of Garcinia Mangostana Xanthones in Eudragit RL100/RS100 for Anti-Colon Cancer Drug Delivery. J. Nanomater. 2015, 2015, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Zak, A.; Majid, W.; ME, A.; Yousefi, R. X-ray Analysis of ZnO Nanoparticles by Williamson–Hall and Size–Strain Plot Methods. Solid State Sci. 2011, 13, 251. [Google Scholar] [CrossRef]
- CCRC, U. Standard Procedure of the Cytotoxic Test MTT Method. Cancer Chemoprevention Res. Cent. 2012, 1, 1–7. [Google Scholar]
- Guo, M.; Wang, X.; Lu, X.; Wang, H.; Brodelius, P.E. α-Mangostin Extraction from the Native Mangosteen (Garcinia mangostana L.) and the Binding Mechanisms of α-Mangostin to HSAorTRF. PLoS ONE 2016, 11, e0161566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohman, A.; Arifah, F.H.; Irnawati; Alam, G.; Muchtaridi, M. The Application of FTIR Spectroscopy and Chemometrics for Classification of Mangosteen Extract and Its Correlation with Alpha-Mangostin. J. Appl. Pharm. Sci. 2020, 10, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.; Butt, M.H.; Siddique, W.; Iqbal, M.O.; Nisar, N.; Mumtaz, A.; Nazeer, H.Y.; Alshammari, A.; Riaz, M.S. Fabrication of PEGylated Chitosan Nanoparticles Containing Tenofovir Alafenamide: Synthesis and Characterization. Molecules 2022, 27, 8401. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Chun, S.C.; Chandrasekaran, M. Preparation and in Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, J.; Döll-Boscardin, P.M.; Fiorin, B.C.; Nadal, J.M.; Farago, P.V.; De Paula, J.P. Development and Characterization of Hyaluronic Acid-Lysine Nanoparticles with Potential as Innovative Dermal Filling. Braz. J. Pharm. Sci. 2016, 52, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Pandey, M.; Choudhury, H.; Ying, P.C.; Xian, T.M.; Kaur, T.; Jia, G.W.; Gorain, B. Hyaluronic Acid Functionalized Nanoparticles for Simultaneous Delivery of Curcumin and Resveratrol for Management of Chronic Diabetic Wounds: Fabrication, Characterization, Stability and in Vitro Release Kinetics. J. Drug Deliv. Sci. Technol. 2020, 57, 101747. [Google Scholar] [CrossRef]
- Iqbal, A.; Muhammad Shuib, N.A.; Darnis, D.S.; Miskam, M.; Abdul Rahman, N.R.; Adam, F. Synthesis and Characterisation of Rice Husk Ash Silica Drug Carrier for α-Mangostin. J. Phys. Sci. 2018, 29, 95–107. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen Pericarp Extract Embedded in Electrospun PVP Nanofiber Mats: Physicochemical Properties and Release Mechanism of α-Mangostin. Int. J. Nanomed. 2018, 13, 4927–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulia, K.; Rachman, D.; Krisanti, E.A. Preparation, Characterization and Release Profile of Chitosan Alginate Freeze Dried Matrices Loaded with Mangostins. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1295. [Google Scholar] [CrossRef]
- Eddya, M.; Tbib, B.; EL-Hami, K. A Comparison of Chitosan Properties after Extraction from Shrimp Shells by Diluted and Concentrated Acids. Heliyon 2020, 6, e03486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgorbunskikh, E.; Kuskov, T.; Rychkov, D.; Lomovskii, O.; Bychkov, A. Mechanical Amorphization of Chitosan with Different Molecular Weights. Polymers 2022, 14, 4438. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and Development of Ligand-Appended Polysaccharidic Nanoparticles for the Delivery of Oxaliplatin in Colorectal Cancer. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 179–190. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of Active Targeting Nanoparticle Delivery System for Chemotherapeutic Drugs and Traditional/Herbal Medicines in Cancer Therapy: A Systematic Review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, J.; Varas-Godoy, M.; Haidar, Z.S. Physicochemical Characterization of Chitosan-Hyaluronan-Coated Solid Lipid Nanoparticles for the Targeted Delivery of Paclitaxel: A Proof-of-Concept Study in Breast Cancer Cells. Nanomedicine 2017, 12, 473–490. [Google Scholar] [CrossRef]
- Almeida, P.V.; Shahbazi, M.A.; Mäkilä, E.; Kaasalainen, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. Amine-Modified Hyaluronic Acid-Functionalized Porous Silicon Nanoparticles for Targeting Breast Cancer Tumors. Nanoscale 2014, 6, 10377–10387. [Google Scholar] [CrossRef] [Green Version]
- Almalik, A.; Karimi, S.; Ouasti, S.; Donno, R.; Wandrey, C.; Day, P.J.; Tirelli, N. Hyaluronic Acid (HA) Presentation as a Tool to Modulate and Control the Receptor-Mediated Uptake of HA-Coated Nanoparticles. Biomaterials 2013, 34, 5369–5380. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via Epr Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Conti, B.; Modena, T.; Cova, E.; Meloni, F.; Genta, I. Hyaluronic Acid-Decorated Chitosan Nanoparticles for CD44-Targeted Delivery of Everolimus. Int. J. Mol. Sci. 2018, 19, 2310. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.M.; Melo, M.N.; Santos, Á.K.M.; Oliveira, K.V.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; Fricks, A.T. Hyaluronic Acid-Coated Chitosan Nanoparticles as Carrier for the Enzyme/Prodrug Complex Based on Horseradish Peroxidase/Indole-3-Acetic Acid: Characterization and Potential Therapeutic for Bladder Cancer Cells. Enzym. Microb. Technol. 2021, 150, 109889. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of Alpha Mangostin-Loaded Chitosan/Alginate Controlled-Release Nanoparticles Using Genipin as Crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Polexe, R.C.; Delair, T. Elaboration of Stable and Antibody Functionalized Positively Charged Colloids by Polyelectrolyte Complexation between Chitosan and Hyaluronic Acid. Molecules 2013, 18, 8563–8578. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug Release Study of the Chitosan-Based Nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, E.; Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, E.; Mangin, D.; Ofridam, F.; et al. PH-Sensitive Polymers: Classification and Some Fine Potential Applications To Cite This Version: HAL Id: Hal-03132353 PH-Sensitive Polymers: Classification and Some Fine Potential Applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Bai, X.; Smith, Z.L.; Wang, Y.; Butterworth, S.; Tirella, A. Sustained Drug Release from Smart Nanoparticles in Cancer Therapy: A Comprehensive Review. Micromachines 2022, 13, 1623. [Google Scholar] [CrossRef]
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. [Google Scholar] [CrossRef]
- Azadi, A.; Hamidi, M.; Khoshayand, M.R.; Amini, M.; Rouini, M.R. Preparation and Optimization of Surface-Treated Methotrexate-Loaded Nanogels Intended for Brain Delivery. Carbohydr. Polym. 2012, 90, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Hyaluronated Nanoparticles with PH- and Enzyme-Responsive Drug Release Properties. Colloids Surfaces B Biointerfaces 2014, 116, 359–364. [Google Scholar] [CrossRef]
- Ullah, F.; Shah, K.U.; Shah, S.U.; Nawaz, A.; Nawaz, T.; Khan, K.A.; Alserihi, R.F.; Tayeb, H.H.; Tabrez, S.; Alfatama, M. Synthesis, Characterization and In Vitro Evaluation of Chitosan Nanoparticles Physically Admixed with Lactose Microspheres for Pulmonary Delivery of Montelukast. Polymers 2022, 14, 3564. [Google Scholar] [CrossRef]
- Saleh, A.; Akkuş-Dağdeviren, Z.B.; Friedl, J.D.; Knoll, P.; Bernkop-Schnürch, A. Chitosan—Polyphosphate Nanoparticles for a Targeted Drug Release at the Absorption Membrane. Heliyon 2022, 8, e10577. [Google Scholar] [CrossRef]
- Manimaran, D.; Elangovan, N.; Mani, P.; Subramanian, K.; Ali, D.; Alarifi, S.; Palanisamy, C.P.; Zhang, H.; Rangasamy, K.; Palanisamy, V.; et al. Isolongifolene-Loaded Chitosan Nanoparticles Synthesis and Characterization for Cancer Treatment. Sci. Rep. 2022, 12, 19250. [Google Scholar] [CrossRef]
- Waqas, M.K.; Safdar, S.; Buabeid, M.; Ashames, A.; Akhtar, M.; Murtaza, G. Alginate-Coated Chitosan Nanoparticles for PH-Dependent Release of Tamoxifen Citrate. J. Exp. Nanosci. 2022, 17, 522–534. [Google Scholar] [CrossRef]
- Tapdiqov, S.; Taghiyev, D.; Zeynalov, N.; Safaraliyeva, S.; Fatullayeva, S.; Hummetov, A.; Raucci, M.; Mustafayev, M.; Jafarova, R.; Shirinova, K. Cumulative Release Kinetics of Levothyroxine-Na Pentahydrate from Chitosan/Arabinogalactane Based PH Sensitive Hydrogel and It’s Toxicology. React. Funct. Polym. 2022, 178, 105334. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Cytotoxicity Enhancement in MCF-7 Breast Cancer Cells with Depolymerized Chitosan Delivery of α-Mangostin. Polymers 2022, 14, 3139. [Google Scholar] [CrossRef]






| Formulation | AM (mg/mL) | CS (mg/mL) | TPP (mg/mL) | HA (mg/mL) |
|---|---|---|---|---|
| AM-CS | 1 | 10 | 2 | - |
| AM-CS/HA1 | 1 | 10 | 2 | 20 |
| AM-CS/HA2 | 1 | 10 | 2 | 40 |
| AM-CS/HA3 | 1 | 10 | 2 | 60 |
| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| AM-CS | 229.133 ± 5.685 | 0.382 ± 0.015 | 33.83 ± 1.92 |
| AM-CS/HA1 | 304.833 ± 6.288 | 0.362 ± 0.038 | −24.43 ± 1.76 |
| AM-CS/HA2 | 369.300 ± 2.467 | 0.360 ± 0.028 | −28.44 ± 2.26 |
| AM-CS/HA3 | 412.767 ± 6.001 | 0.346 ± 0.034 | −33.31 ± 1.85 |
| Formulation | EE (%) | DL (%) |
|---|---|---|
| AM-CS | 88.325 ± 3.340 | 8.674 ± 0.018 |
| AM-CS/HA1 | 90.404 ± 2.161 | 8.514 ± 0.007 |
| Parameter | AM-CS | AM-CS/HA1 | ||
|---|---|---|---|---|
| pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | |
| Intercept | 0.852 ± 0.477 | 3.265 ± 0.129 | 0.889 ± 0.169 | 1.723 ± 0.3428 |
| Slope | 9.281 ± 0.489 | 11.821 ± 0.377 | 9.097 ± 0.160 | 10.289 ± 0.340 |
| Correlation coefficient (r) | 0.994 ± 0.002 | 0.981 ± 0.005 | 0.995 ± 0.001 | 0.987 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meylina, L.; Muchtaridi, M.; Joni, I.M.; Elamin, K.M.; Wathoni, N. Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells. Polymers 2023, 15, 1025. https://doi.org/10.3390/polym15041025
Meylina L, Muchtaridi M, Joni IM, Elamin KM, Wathoni N. Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells. Polymers. 2023; 15(4):1025. https://doi.org/10.3390/polym15041025
Chicago/Turabian StyleMeylina, Lisna, Muchtaridi Muchtaridi, I Made Joni, Khaled M. Elamin, and Nasrul Wathoni. 2023. "Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells" Polymers 15, no. 4: 1025. https://doi.org/10.3390/polym15041025
APA StyleMeylina, L., Muchtaridi, M., Joni, I. M., Elamin, K. M., & Wathoni, N. (2023). Hyaluronic Acid-Coated Chitosan Nanoparticles as an Active Targeted Carrier of Alpha Mangostin for Breast Cancer Cells. Polymers, 15(4), 1025. https://doi.org/10.3390/polym15041025