Influence of the Chemical Structure on the Mechanical Relaxation of Dendrimers
Abstract
:1. Introduction
2. Simulation Details and Theoretical Approach
3. Results and Discussions
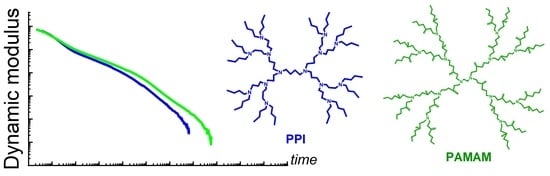
3.1. Relaxation Modulus
3.2. Storage and Loss Moduli
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Buhleier, E.; Wehner, W.; Voegtle, F. “Cascade”-And “Nonskid-chain-like” Syntheses of Molecular Cavity Topologies. Synthesis Stuttg. 1978, 2, 155–158. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C. Dendrimers Derived from 1 → 3 Branching Motifs. Chem. Rev. 2010, 110, 6338–6442. [Google Scholar] [PubMed]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef] [PubMed]
- Markelov, D.A.; Semisalova, A.S.; Mazo, M.A. Formation of a Hollow Core in Dendrimers in Solvents. Macromol. Chem. Phys. 2021, 222, 2100085. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef]
- Sowinska, M.; Urbanczyk-Lipkowska, Z. Advances in the chemistry of dendrimers. New J. Chem. 2014, 38, 2168. [Google Scholar] [CrossRef]
- Kang, T.; Amir, R.J.; Khan, A.; Ohshimizu, K.; Hunt, J.N.; Sivanandan, K.; Montañez, M.I.; Malkoch, M.; Ueda, M.; Hawker, C.J. Facile access to internally functionalized dendrimers through efficient and orthogonal click reactions. Chem. Commun. 2010, 46, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S. Functionalizing the interior of dendrimers: Synthetic challenges and applications. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1047–1058. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Gorzkiewicz, M.; Konopka, M.; Janaszewska, A.; Tarasenko, I.I.; Sheveleva, N.N.; Gajek, A.; Neelov, I.M.; Klajnert-Maculewicz, B. Application of new lysine-based peptide dendrimers D3K2 and D3G2 for gene delivery: Specific cytotoxicity to cancer cells and transfection in vitro. Bioorg. Chem. 2020, 95, 103504. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Bugno, J.; Lee, S.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. WIREs Nanomed. Nanobiotechnology 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as Drug Carriers: Applications in Different Routes of Drug Administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Salunkhe, S.; Agrawal, M.; Kali, M.; Singhvi, G.; Tiwari, S.; Saraf, S.; Saraf, S.; Alexander, A. Understanding the Pharmaceutical Aspects of Dendrimers for the Delivery of Anticancer Drugs. Curr. Drug Targets 2020, 21, 528–540. [Google Scholar] [CrossRef]
- Kuang, T.; Chang, L.; Fu, D.; Yang, J.; Zhong, M.; Chen, F.; Peng, X. Improved crystallizability and processability of ultra high molecular weight polyethylene modified by poly(amido amine) dendrimers. Polym. Eng. Sci. 2017, 57, 153–160. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, B.; Nian, F.; Zhao, Y.; Wang, K.; Pang, H. Synthesis and characterization of a PAMAM dendrimer-based superplasticizer and its effect on the properties in cementitious system. J. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Gotlib, Y.Y.; Markelov, D.A. Theory of the Relaxation Spectrum of a Dendrimer Macromolecule. Polym. Sci. Ser. A 2002, 44, 1341–1350. [Google Scholar]
- Cai, C.; Chen, Z.Y. Rouse Dynamics of a Dendrimer Model in the ϑ Condition. Macromolecules 1997, 30, 5104–5117. [Google Scholar] [CrossRef]
- Grimm, J.; Dolgushev, M. Dynamics of internally functionalized dendrimers. Phys. Chem. Chem. Phys. 2016, 18, 19050–19061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, P.; Kant, R.; Blumen, A. Polymer dynamics and topology: Extension of stars and dendrimers in external fields. Macromol. Theory Simul. 2000, 9, 56–67. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Markelov, D.A.; Gotlib, Y.Y.; Blumen, A. Dynamics of dendrimer-based polymer networks. J. Chem. Phys. 2003, 119, 7579–7590. [Google Scholar] [CrossRef]
- Blumen, A.; Gurtovenko, A.A. Generalized Gaussian Structures: Models for Polymer Systems with Complex Topologies. Adv. Polym. Sci. 2005, 182, 171–282. [Google Scholar]
- Dolgushev, M.; Blumen, A. Dynamics of semiflexible treelike polymeric networks. J. Chem. Phys. 2009, 131, 044905. [Google Scholar] [CrossRef] [PubMed]
- Dolgushev, M.; Blumen, A. Dynamics of Semiflexible Chains, Stars, and Dendrimers. Macromolecules 2009, 42, 5378–5387. [Google Scholar] [CrossRef]
- Markelov, D.A.; Lähderanta, E.; Gotlib, Y.Y. Influence of Modified Terminal Segments on Dynamic Modulus and Viscosity of Dendrimer. Macromol. Theory Simul. 2010, 19, 158–169. [Google Scholar] [CrossRef]
- Dolgushev, M.; Blumen, A. Dynamics of chains and dendrimers with heterogeneous semiflexibility. J. Chem. Phys. 2010, 132, 124905. [Google Scholar] [CrossRef]
- Dolgushev, M.; Berezovska, G.; Blumen, A. Branched semiflexible polymers: Theoretical and simulation aspects. Macromol. Theory Simulations 2011, 20, 621–644. [Google Scholar] [CrossRef]
- Uppuluri, S.; Morrison, F.A.; Dvornic, P.R. Rheology of dendrimers. 2. Bulk polyamidoamine dendrimers under steady shear, creep, and dynamic oscillatory shear. Macromolecules 2000, 33, 2551–2560. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Ahn, D.U. Processability of Hyperbranched Poly(ether ketone)s with Different Degrees of Branching from Viewpoints of Molecular Mobility and Comparison with Their Linear Analogue. Macromolecules 2000, 33, 7557–7563. [Google Scholar] [CrossRef]
- Wu, W.; Driessen, W.; Jiang, X. Oligo(ethylene glycol)-Based Thermosensitive Dendrimers and Their Tumor Accumulation and Penetration. J. Am. Chem. Soc. 2014, 136, 3145–3155. [Google Scholar] [CrossRef] [PubMed]
- Milenin, S.A.; Selezneva, E.V.; Tikhonov, P.A.; Vasil’ev, V.G.; Buzin, A.I.; Balabaev, N.K.; Kurbatov, A.O.; Petoukhov, M.V.; Shtykova, E.V.; Feigin, L.A.; et al. Hybrid Polycarbosilane-Siloxane Dendrimers: Synthesis and Properties. Polymers 2021, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Gainaru, C.; Cetinkaya, B.; Valiullin, R.; Fatkullin, N.; Rössler, E.A. Field-Cycling Relaxometry as a Molecular Rheology Technique: Common Analysis of NMR, Shear Modulus and Dielectric Loss Data of Polymers vs Dendrimers. Macromolecules 2015, 48, 7521–7534. [Google Scholar] [CrossRef]
- Mohamed, F.; Hofmann, M.; Pötzschner, B.; Fatkullin, N.; Rössler, E.A. Dynamics of PPI Dendrimers: A Study by Dielectric and H-2 NMR Spectroscopy and by Field-Cycling H-1 NMR Relaxometry. Macromolecules 2015, 48, 3294–3302. [Google Scholar] [CrossRef]
- Vasil’ev, V.G.; Kramarenko, E.Y.; Tatarinova, E.A.; Milenin, S.A.; Kalinina, A.A.; Papkov, V.S.; Muzafarov, A.M. An unprecedented jump in the viscosity of high-generation carbosilane dendrimer melts. Polymer Guildf. 2018, 146, 1–5. [Google Scholar] [CrossRef]
- Sendijarevic, I.; McHugh, A.J. Effects of Molecular Variables and Architecture on the Rheological Behavior of Dendritic Polymers. Macromolecules 2000, 33, 590–596. [Google Scholar] [CrossRef]
- Matveev, V.V.; Markelov, D.A.; Dvinskikh, S.V.; Shishkin, A.N.; Tyutyukin, K.V.; Penkova, A.V.; Tatarinova, E.A.; Ignat’Eva, G.M.; Milenin, S.A. Investigation of Melts of Polybutylcarbosilane Dendrimers by 1H NMR Spectroscopy. Sci. Rep. 2017, 7, 13710. [Google Scholar] [CrossRef]
- Karatasos, K. Static and Dynamic Behavior in Model Dendrimer Melts: Toward the Glass Transition. Macromolecules 2005, 38, 4472–4483. [Google Scholar] [CrossRef]
- Dolgushev, M.; Markelov, D.A.; Lähderanta, E. Linear Viscoelasticity of Carbosilane Dendrimer Melts. Macromolecules 2019, 52, 2542–2547. [Google Scholar] [CrossRef]
- Balabaev, N.K.; Mazo, M.A.; Kramarenko, E.Y. Insight into the Structure of Polybutylcarbosilane Dendrimer Melts via Extensive Molecular Dynamics Simulations. Macromolecules 2017, 50, 432–445. [Google Scholar] [CrossRef]
- Bag, S.; Jain, M.; Maiti, P.K. Charge Transport in Dendrimer Melts Using Multiscale Modeling Simulation. J. Phys. Chem. B 2016, 120, 9142–9151. [Google Scholar] [CrossRef] [Green Version]
- Markelov, D.A.; Shishkin, A.N.; Matveev, V.V.; Penkova, A.V.; Lähderanta, E.; Chizhik, V.I. Orientational Mobility in Dendrimer Melts: Molecular Dynamics Simulations. Macromolecules 2016, 49, 9247–9257. [Google Scholar] [CrossRef]
- Smeijers, A.F.; Markvoort, A.J.; Pieterse, K.; Hilbers, P.A.J. Coarse-grained simulations of poly(propylene imine) dendrimers in solution. J. Chem. Phys. 2016, 144, 074903. [Google Scholar] [CrossRef] [PubMed]
- Kurbatov, A.O.; Balabaev, N.K.; Mazo, M.A.; Kramarenko, E.Y. Effects of generation number, spacer length and temperature on the structure and intramolecular dynamics of siloxane dendrimer melts: Molecular dynamics simulations. Soft Matter 2020, 16, 3792–3805. [Google Scholar] [CrossRef]
- Gotlib, Y.Y.; Markelov, D.A. Permittivity of a dendrimer containing polar groups. Polym. Sci. Ser. A 2004, 46, 815–832. [Google Scholar]
- Gotlib, Y.Y.; Markelov, D.A. Theory of orientational relaxation of individual specified units in a dendrimer. Polym. Sci. Ser. A 2007, 49, 1137–1154. [Google Scholar] [CrossRef]
- Markelov, D.A.; Dolgushev, M.; Gotlib, Y.Y.; Blumen, A. NMR relaxation of the orientation of single segments in semiflexible dendrimers. J. Chem. Phys. 2014, 140, 244904. [Google Scholar] [CrossRef]
- Markelov, D.A.; Dolgushev, M.; Lähderanta, E. Chapter One—NMR Relaxation in Dendrimers. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 91, pp. 1–66. ISBN 0066-4103. [Google Scholar]
- Shishkin, A.N.; Markelov, D.A.; Matveev, V.V. Molecular dynamics simulation of poly(butyl)carbosilane dendrimer melts at 600 K. Russ. Chem. Bull. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Bosko, J.T.; Todd, B.D.; Sadus, R.J. Viscoelastic properties of dendrimers in the melt from nonequlibrium molecular dynamics. J. Chem. Phys. 2004, 121, 12050–12059. [Google Scholar] [CrossRef] [PubMed]
- Bosko, J.T.; Todd, B.D.; Sadus, R.J. Molecular simulation of dendrimers and their mixtures under shear: Comparison of isothermal-isobaric (NpT) and isothermal-isochoric (NVT) ensemble systems. J. Chem. Phys. 2005, 123, 034905. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, E.; Todd, B.D.; Daivis, P.J. Nonequilibrium molecular dynamics simulation of dendrimers and hyperbranched polymer melts undergoing planar elongational flow. J. Rheol. 2014, 58, 281–305. [Google Scholar] [CrossRef]
- Sheveleva, N.N.; Dolgushev, M.; Lähderanta, E.; Markelov, D.A. Mechanical relaxation of functionalized carbosilane dendrimer melts. Phys. Chem. Chem. Phys. 2022, 24, 13049–13056. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Osterwinter, C.; Tonhauser, C.; Schömer, M.; Wilms, D.; Frey, H.; Friedrich, C. Can hyperbranched polymers entangle? Effect of hydrogen bonding on entanglement transition and thermorheological properties of hyperbranched polyglycerol melts. Macromolecules 2016, 49, 8722–8737. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Matsui, M.; Akaogi, M. Molecular Dynamics Simulation of the Structural and Physical Properties of the Four Polymorphs of TiO2. Mol. Simul. 1991, 6, 239–244. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Ramírez, J.; Sukumaran, S.K.; Vorselaars, B.; Likhtman, A.E. Efficient on the fly calculation of time correlation functions in computer simulations. J. Chem. Phys. 2010, 133, 154103. [Google Scholar] [CrossRef]
- David, A.; De Nicola, A.; Tartaglino, U.; Milano, G.; Raos, G. Viscoelasticity of Short Polymer Liquids from Atomistic Simulations. J. Electrochem. Soc. 2019, 166, B3246–B3256. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Structure and properties of rigid chain polymer molecules in solutions. Polym. Sci. U.S.S.R. 1979, 21, 2879–2899. [Google Scholar] [CrossRef]
- Gury, L.; Gauthier, M.; Cloitre, M.; Vlassopoulos, D. Colloidal Jamming in Multiarm Star Polymer Melts. Macromolecules 2019, 52, 4617–4623. [Google Scholar] [CrossRef]






| Dendrimer | Nter | Md, g/mol | Rg, nm | <NHB> | τrot, ns | τmax, ns | τrot/Rg3, ns nm−3 | τmax/τrot | |
|---|---|---|---|---|---|---|---|---|---|
| intra | inter | ||||||||
| PAMAM | 16 | 3256.2 | 1.034 | 10.9 | 7.5 | 7.916 | 4.088 | 7.16 | 0.52 |
| PPI | 16 | 1686.8 | 0.873 | 2 | 0 | 1.074 | 0.512 | 1.61 | 0.46 |
| PCS | 16 | 1964.3 | 0.872 | 0 | 0 | 0.831 | 0.461 | 1.25 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheveleva, N.N.; Komolkin, A.V.; Markelov, D.A. Influence of the Chemical Structure on the Mechanical Relaxation of Dendrimers. Polymers 2023, 15, 833. https://doi.org/10.3390/polym15040833
Sheveleva NN, Komolkin AV, Markelov DA. Influence of the Chemical Structure on the Mechanical Relaxation of Dendrimers. Polymers. 2023; 15(4):833. https://doi.org/10.3390/polym15040833
Chicago/Turabian StyleSheveleva, Nadezhda N., Andrei V. Komolkin, and Denis A. Markelov. 2023. "Influence of the Chemical Structure on the Mechanical Relaxation of Dendrimers" Polymers 15, no. 4: 833. https://doi.org/10.3390/polym15040833
APA StyleSheveleva, N. N., Komolkin, A. V., & Markelov, D. A. (2023). Influence of the Chemical Structure on the Mechanical Relaxation of Dendrimers. Polymers, 15(4), 833. https://doi.org/10.3390/polym15040833