Thiophene End-Functionalized Oligo-(D,L-Lactide) as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
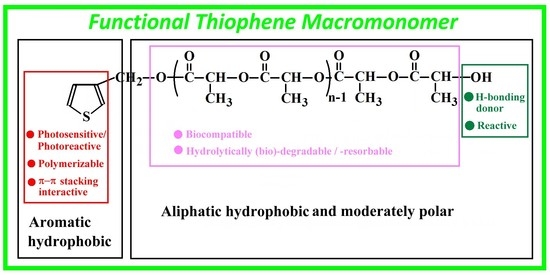
2.2. Synthesis
2.2.1. Synthesis of PLA-Based Macromonomer (Th-PDLLA) by Ring-Opening-Polymerization (ROP)
2.2.2. Synthesis of OTh-PDDLA by Photochemical Oxidative Polymerization of Th-PDLLA Macromonomer
2.3. Measurements
3. Results and Discussion
3.1. Synthesis and Structural Characterization of Th-PDLLA

3.2. Properties of Th-PDLLA in Solution and in Bulk
3.2.1 The Behaviour of Th-PDLLA in Different Organic Solvents
3.2.2. Thermal Properties of Th-PDLLA
3.3. Preliminary Results of Metal-Free Photoinduced Oxidative Homopolymerization of Th-PDLLA


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Amin, K.; An, Z.; Cai, Z.; Chen, H.; Chen, H.; Dong, Y.; Feng, X.; Fu, W.; Gu, J.; et al. Advanced functional polymer materials. Mater. Chem. Front. 2020, 4, 1803–1915. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Zhang, Y.; Li, C.; He, Z.; Choy, W.C.H.; Low, P.J.; Sonar, P.; Kyaw, A.K.K. Biodegradable Materials and Green Processing for Green Electronics. Adv. Mater. 2020, 32, 200159. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Jiang, K.; Shen, G. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev. 2017, 46, 6764–6815. [Google Scholar] [CrossRef]
- Sun, Q.; Qian, B.; Uto, K.; Chen, J.; Liua, X.; Minari, T. Functional biomaterials towards flexible electronics and sensors. Biosens. Bioelectron. 2018, 119, 237–251. [Google Scholar] [CrossRef]
- Wang, S.; Oh, J.Y.; Xu, J.; Tran, H.; Bao, Z. Skin-Inspired Electronics: An Emerging Paradigm. Acc. Chem. Res. 2018, 51, 1033–1045. [Google Scholar] [CrossRef]
- Tran, H.; Feig, V.R.; Liu, K.; Zheng, Y.; Bao, Z. Polymer Chemistries Underpinning Materials for Skin-Inspired Electronics. Macromolecules 2019, 52, 3965–3974. [Google Scholar] [CrossRef] [Green Version]
- Skabara, P.J. A brief perspective on the evolution of plastic electronics—From highly conducting polymers to conjugated organic semiconductors. Chem. Commun. 2013, 49, 9242–9244. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to applications. Nat. Mat. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Hsieh, H.-C.; Wu, N.; Chuang, T.-H.; Lee, W.-Y.; Chen, J.-Y.; Chen, W.-C. Eco-Friendly Polyfluorene/Poly(butylene succinate) Blends and Their Electronic Device Application on Biodegradable Substrates. ACS Appl. Polym. Mater. 2020, 2, 2469–2476. [Google Scholar] [CrossRef]
- Keersmaecker, M.D.; Lang, A.W.; Österholm, A.M.; Reynolds, J.R. All Polymer Solution Processed Electrochromic Devices: A Future without Indium Tin Oxide. ACS Appl. Mater. Interfaces 2018, 10, 31568–31579. [Google Scholar] [CrossRef]
- Stucchia, E.; Maksimovica, K.; Bertolacci, L.; Viola, F.A.; Athanassiou, A.; Caironi, M. Biodegradable all-polymer field-effect transistors printed on Mater-Bi. J. Inf. Disp. 2021, 22, 247–256. [Google Scholar] [CrossRef]
- Rao, Z.; Thukral, A.; Yang, P.; Lu, Y.; Shim, H.; Wu, W.; Karim, A.; Yu, C. All-Polymer Based Stretchable Rubbery Electronics and Sensors. Adv. Funct. Mater. 2022, 32, 2111232. [Google Scholar] [CrossRef]
- Fahlman, M.; Fabiano, S.; Gueskine, V.; Simon, D.; Berggren, M.; Crispin, X. Interfaces in organic electronics. Nat. Rev. Mater. 2019, 4, 627–650. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, W.; Sirringhaus, H.; Müllen, K. Recent Progress in Emerging Organic Semiconductors. Adv. Mater. 2022, 34, 2108701. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Mei, J. What’s next for semiconducting polymers. J. Polym. Sci. 2022, 60, 287–289. [Google Scholar] [CrossRef]
- Azhou, Y. Conjugated polymers for functional applications. Polym. Int. 2021, 70, 357. [Google Scholar]
- Toribio, F.O. Biomimetic Conducting Polymers: Synthesis, Materials, Properties, Functions, and Devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotech. 2013, 24, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, B.D.; Fabiano, S.; Rivnay, J. Mixed Ionic Electronic Transport in Polymers. Annu. Rev. Mater. Res. 2021, 51, 73–99. [Google Scholar] [CrossRef]
- Kenry; Liu, B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef]
- Tropp, J.; Rivnay, J. Design of biodegradable and biocompatible conjugated polymers for bioelectronics. J. Mater. Chem. C 2021, 9, 13543–13556. [Google Scholar] [CrossRef]
- Baker, C.; Wagner, K.; Wagner, P.; Officer, D.L.; Mawad, D. Biofunctional conducting polymers: Synthetic advances, challenges, and perspectives towards their use in implantable bioelectronic devices. Adv. Phys.-X 2021, 6, 1899850. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bao, Z. Biomaterials-based organic electronic devices. Polym. Int. 2010, 59, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Du, X.; Long, Y.; Zheng, H. Biodegradable polymeric materials for flexible and degradable electronics. Front. Electron. 2022, 3, 985681. [Google Scholar] [CrossRef]
- Feinera, R.; Fleischer, S.; Shapira, A.; Kalish, O.; Dvir, T. Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J. Control. Release 2018, 281, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Bendrea, A.-D.; Fabregat, G.; Cianga, L.; Estrany, F.; del Valle, L.J.; Cianga, I.; Aleman, C. Hybrid materials consisting of an all-conjugated polythiophene backbone and grafted hydrophilic poly(ethylene glycol) chains. Polym. Chem. 2013, 4, 2709–2723. [Google Scholar] [CrossRef]
- Molina, B.G.; Bendrea, A.D.; Cianga, L.; Armelin, E.; del Valle, L.J.; Cianga, I.; Alemán, C. The biocompatible polythiophene-g-polycaprolactone copolymer as an efficient dopamine sensor platform. Polym. Chem. 2017, 8, 6112–6122. [Google Scholar] [CrossRef] [Green Version]
- Molina, B.G.; Cianga, L.; Bendrea, A.-D.; Cianga, I.; Alemán, C.; Armelin, E. An amphiphilic, heterografted polythiophene copolymer containing biocompatible/biodegradable side chains for use as an (electro)active surface in biomedical applications. Polym. Chem. 2019, 10, 5010–5022. [Google Scholar] [CrossRef]
- Molina, B.G.; Bendrea, A.-D.; Lanzalaco, S.; Franco, L.; Cianga, L.; del Valle, L.J.; Puiggali, J.; Turon, P.; Armelin, E.; Cianga, I.; et al. Smart design for a flexible, functionalized an electroresponsive hybrid platform based on poly(3,4-ethylenedioxythiophene) derivatives to improve cell viability. J. Mater. Chem. B 2020, 8, 8864–8877. [Google Scholar] [CrossRef]
- Sengel, T.Y.; Guler, E.; Gumus, Z.P.; Aldemir, E.; Coskunol, H.; Akbulut, H.; Colak, D.G.; Cianga, I.; Yamada, S.; Timur, S.; et al. An immunoelectrochemical platform for the biosensing of ‘Cocaine use’. Sens. Actuators B 2017, 246, 310–318. [Google Scholar] [CrossRef]
- da Silva, A.C.; Augusto, T.; Andrade, L.H.; de Torresi, S.I.C. One pot biocatalytic synthesis of a biodegradable electroactive macromonomer based on 3,4-ethylenedioxytiophene and poly(L-lactic acid). Mater. Sci. Eng. C 2018, 83, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Domagala, A.; Domagala, W.; Ledwon, P.; Musiol, M.; Janeczek, H.; Stolarczyk, A.; Kurcok, P.; Adamus, G.; Lapkowski, M. N-Oligo(3-hydroxybutyrate)-functionalized polypyrroles: Towards bio-erodible conducting copolymers. Polym. Int. 2016, 65, 1395–1404. [Google Scholar] [CrossRef]
- Ritzau-Reid, K.I.; Spicer, C.D.; Gelmi, A.; Grigsby, C.L.; Ponder, J.F., Jr.; Bemmer, V.; Creamer, A.; Vilar, R.; Serio, A.; Stevens, M.M. An Electroactive Oligo-EDOT Platform for Neural Tissue Engineering. Adv. Funct. Mater. 2020, 30, 2003710. [Google Scholar] [CrossRef]
- Jiang, D.-H.; Ree, B.J.; Isono, T.; Xia, X.-C.; Hsu, L.-C.; Kobayashi, S.; Ngoi, K.H.; Chen, W.-C.; Jao, C.-C.; Veeramuthu, L.; et al. Facile one-pot synthesis of rod-coil bio-block copolymers and uncovering their role in forming the efficient stretchable touch-responsive light emitting diodes. Chem. Eng. J. 2021, 418, 129421. [Google Scholar] [CrossRef]
- Nikkhah, M.; Rivnay, J. Conductive and Electroactive Biomaterials and Bioelectronics. Acta Biomat. 2022, 139, 1–3. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, L.-W.; Wu, B.-H.; Xu, Z.-K.; Wan, L.-S. End-functionalized polymers by controlled/living radical polymerizations: Synthesis and applications. Polym. Chem. 2022, 13, 300–358. [Google Scholar] [CrossRef]
- Chanthaset, N.; Ajiro, H. Synthetic Biodegradable Polymers with Chain End Modification: Polylactide, Poly(butylene succinate), and Poly(hydroxyalkanoate). Chem. Lett. 2021, 50, 767–777. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, Y.; Jiang, L.; Dan, Y. Polylactide with improved optical property by introducing natural functional substance: Aloe-emodin. React. Func. Polym. 2020, 148, 104486. [Google Scholar] [CrossRef]
- Gorbachuk, V.V.; Padnya, P.L.; Mostovaya, O.A.; Gerasimov, A.V.; Stoikov, I.I. Towards novel functional polymers: Ring-opening polymerization of L-lactide with p-tert-butylthiacalix[4]arene derivatives. React. Func. Polym. 2020, 150, 104546. [Google Scholar] [CrossRef]
- Kost, B.; Basko, M.; Bednarek, M.; Socka, M.; Kopka, B.; Lapienis, G.; Biela, T.; Kubisa, P.; Brzezinski, M. The influence of the functional end groups on the properties of polylactide-based materials. Prog. Polym. Sci. 2022, 130, 101556. [Google Scholar] [CrossRef]
- Bielas, R.; Wrobel-Marek, J.; Kurczynska, E.U.; Neugebauer, D. Pyranine labeled polymer nanoparticles as fluorescent markers forcell wall staining and imaging of movement within apoplast. Sens. Actuators B Chem. 2019, 297, 126789. [Google Scholar] [CrossRef]
- Al-Attar, H.; Alwattar, A.A.; Haddad, A.; Abdullah, B.A.; Quayled, P.; Yeates, S.G. Polylactide-perylene derivative for blue biodegradable organic light-emitting diodes. Polym. Int. 2021, 70, 51–58. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Cianga, L.; Ailiesei, G.-L.; Ursu, E.-L.; Colak, D.G.; Cianga, I. 3,4-Ethylenedioxythiophene (EDOT) End-Group Functionalized Poly-ε-caprolactone (PCL): Self-Assembly in Organic Solvents and Its Coincidentally Observed Peculiar Behavior in Thin Film and Protonated Media. Polymers 2021, 13, 2720. [Google Scholar] [CrossRef]
- Rajak, A.; Das, A. Crystallization-Driven Controlled Two-Dimensional (2D) Assemblies from Chromophore-Appended Poly(L-lactide)s: Highly Efficient Energy Transfer on a 2D Surface. Angew. Chem. Int. Ed. 2022, 61, e202116572. [Google Scholar]
- Bendrea, A.-D.; Cianga, L.; Ailiesei, G.-L.; Colak, D.G.; Popescu, I.; Cianga, I. Thiophene α-Chain-End-Functionalized Oligo(2-methyl-2-oxazoline) as Precursor Amphiphilic Macromonomer for Grafted Conjugated Oligomers/Polymers and as a Multifunctional Material with Relevant Properties for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 7495. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, D. Macromonomers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Michalski, A.; Brzezinski, M.; Lapienis, G.; Biela, T. Star-shaped and branched polylactides: Synthesis, characterization, and properties. Prog. Polym. Sci. 2019, 89, 159–212. [Google Scholar] [CrossRef]
- Yamashita, Y. Modification of polymer surfaces by surface active graft copolymers. In Contemporary Topics in Polymer Science; Bailey, V.J., Tsuruta, T., Eds.; Plenum Press: New York, NY, USA, 1984; Volume 4, pp. 463–477. ISBN 978-1-4615-6745-5. [Google Scholar] [CrossRef]
- Müllner, M.; Müller, A.H.E. Cylindrical polymer brushes–Anisotropic building blocks, unimolecular templates and particulate nanocarriers. Polymer 2016, 98, 389–401. [Google Scholar] [CrossRef]
- Alkan, S.; Toppare, L.; Hepuzer, Y.; Yagci, Y. Block Copolymers of Thiophene-Capped Poly(methyl methacrylate) with Pyrrole. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4218–4225. [Google Scholar] [CrossRef]
- Mecerreyes, D.; Pomposo, J.A.; Bengoetxea, M.; Grande, H. Novel Pyrrole End-Functional Macromonomers Prepared by Ring-Opening and Atom-Transfer Radical Polymerizations. Macromolecules 2000, 33, 5846–5849. [Google Scholar] [CrossRef]
- Papila, O.; Toppare, L.; Yagci, Y.; Cianga, L. Conducting Copolymers of Thiophene-Functionalized Polystyrene. Int. J. Polym. Anal. Charact. 2004, 9, 13–28. [Google Scholar] [CrossRef]
- Sahin, E.; Camurlu, P.; Toppare, L.; Mercore, V.M.; Cianga, I.; Yagci, Y. Synthesis and characterization of thiophene functionalized polystyrene copolymers and their electrochemical properties. Polym. Int. 2005, 54, 1599–1605. [Google Scholar] [CrossRef]
- Cianga, I.; Yagci, Y. New polyphenylene-based macromolecular architectures by using well defined macromonomers synthesized via controlled polymerization methods. Prog. Polym. Sci. 2004, 29, 387–399. [Google Scholar] [CrossRef]
- Hicks, G.E.J.; Li, S.; Obhi, N.K.; Jarrett-Wilkins, C.N.; Seferos, D.S. Programmable Assembly of π-Conjugated Polymers. Adv. Mater. 2021, 33, 2006287. [Google Scholar] [CrossRef]
- Penkalla, N.R.; Klapper, M.; Mullen, K. Highly Charged Conjugated Polymers with Polyphenylene Backbones and Poly(acrylic acid) Side Chains. Macromolecules 2012, 45, 2301–2311. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Cianga, L.; Hitruc, E.G.; Titorencu, I.; Cianga, I. Fluorescent Nanoparticles from “Hairy-Rods”, Water-Self Dispersible Amphiphilic Polythiophenes. Mater. Plast. 2013, 50, 71–78. [Google Scholar]
- Cianga, L.; Bendrea, A.-D.; Fifere, N.; Nita, L.E.; Doroftei, F.; Ag, D.; Seleci, M.; Timur, S.; Cianga, I. Fluorescent micellar nanoparticles by selfassembly of amphiphilic, nonionic and water selfdispersible polythiophenes with “hairy rod” architecture. RSC Adv. 2014, 4, 56385–56405. [Google Scholar] [CrossRef]
- Li, Y.; Nese, A.; Hu, X.; Lebedeva, N.V.; LaJoie, T.W.; Burdynska, J.; Stefan, M.C.; You, W.; Yang, W.; Matyjaszewski, K.; et al. Shifting Electronic Structure by Inherent Tension in Molecular Bottlebrushes with Polythiophene Backbones. ACS Macro Lett. 2014, 3, 738–742. [Google Scholar] [CrossRef]
- Maione, S.; Fabregat, G.; del Valle, L.J.; Bendrea, A.-D.; Cianga, L.; Cianga, I.; Estrany, F.; Aleman, C. Effect of the Graft Ratio on the Properties of Polythiophene-g-poly(ethylene glycol). J. Polym. Sci. Part B Polym. Phys. 2015, 53, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Colak, D.G.; Cianga, I.; Cianga, L.; Yagci, Y. Synthesis and self-assembly of fluorenevinylene alternating copolymers in “Hairy-Rod” architecture: Side chain-mediated tuning of conformation, microstructure and photophysical properties. Des. Monomers Polym. 2016, 19, 508–534. [Google Scholar] [CrossRef] [Green Version]
- Colak, D.G.; Egbe, D.A.M.; Birckner, E.; Yurteri, S.; Cianga, I.; Tekin, E.; Schubert, U.S.; Yagci, Y. Photophysical properties of PPP and PPV derivatives bearing polystyrene or polycaprolactone as side groups. Eur. Polym. J. 2009, 45, 940–945. [Google Scholar] [CrossRef]
- Wang, Y.; Park, J.S.; Leech, J.P.; Miao, S.; Bunz, U.H.F. Poly(aryleneethynylenes) with Orange, Yellow, Green, and Blue Solid-State Fluorescence. Macromolecules 2007, 40, 1843–1850. [Google Scholar] [CrossRef]
- Uyar, T.; Cianga, I.; Cianga, L.; Besenbacher, F.; Yagci, Y. Self-aligned and bundled electrospun fibers prepared from blends of polystyrene (PS) and poly(methyl methacrylate) (PMMA) with a hairy-rod polyphenylene copolymer. Mater. Lett. 2009, 63, 1638–1641. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Alfaro, A.; Gabirondo, E.; Alegret, N.; De León-Almazán, C.M.; Hernandez, R.; Vallejo-Illarramendi, A.; Prato, M.; Mecerreyes, D. 3D Printable Conducting and Biocompatible PEDOT-graft-PLA Copolymers by Direct Ink Writing. Macromol. Rapid Commun. 2021, 42, e2100100. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, X.; Yang, M.; Deng, W.; Fan, Q.; Huang, W. Monodispersed Brush-Like Conjugated Polyelectrolyte Nanoparticles with Efficient and Visualized SiRNA Delivery for Gene Silencing. Biomacromolecules 2013, 14, 3643–3652. [Google Scholar] [CrossRef]
- Molina, B.G.; Cianga, L.; Bendrea, A.-D.; Cianga, I.; del Valle, L.J.; Estrany, F.; Aleman, C.; Armelin, E. Amphiphilic polypyrrole-poly(Schiff base) copolymers with poly(ethylene glycol) side chains: Synthesis, properties and applications. Polym. Chem. 2018, 9, 4218–4232. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.C.; Semeano, A.T.S.; Dourado, A.H.B.; Ulrich, H.; de Torresi, S.I.C. Novel Conducting and Biodegradable Copolymers with Noncytotoxic Properties toward Embryonic Stem Cells. ACS Omega 2018, 3, 5593–5604. [Google Scholar] [CrossRef]
- Zangoli, M.; DiMaria, F. Synthesis, characterization, and biological applications of semiconducting polythiophene-based nanoparticles. View 2021, 2, 20200086. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Camurlu, P. Review—Functional Platforms for (Bio)sensing: Thiophene-Pyrrole Hybrid Polymers. J. Electrochem. Soc. 2020, 167, 037557. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Fabregat, G.; Torras, J.; Maione, S.; Cianga, L.; del Valle, L.J.; Cianga, I.; Aleman, C. Polythiophene-g-poly(ethylene glycol) graft copolymers for electroactive scaffolds. J. Mater. Chem. B 2013, 1, 4135–4145. [Google Scholar] [CrossRef] [Green Version]
- Aydin, M.; Aydin, E.B.; Sezginturk, M.K. A Highly Selective Poly(thiophene)-graft-Poly(methacrylamide) Polymer Modified ITO Electrode for Neuron Specific Enolase Detection in Human Serum. Macromol. Biosci. 2019, 19, 1900109. [Google Scholar] [CrossRef]
- Yilmaz, T.; Guler, E.; Gumus, Z.P.; Akbulut, H.; Aldemir, E.; Coskunol, H.; Colak, D.G.; Cianga, I.; Yamada, S.; Timur, S.; et al. Synthesis and application of a novel poly- L-phenylalanine electroactive macromonomer as matrix for the biosensing of ‘Abused Drug’ model. Polym. Chem. 2016, 7, 7304–7315. [Google Scholar] [CrossRef]
- Yagci, Y.; Yilmaz, F.; Kiralp, S.; Toppare, L. Photoinduced Polymerization of Thiophene Using Iodonium Salt. Macromol. Chem. Phys. 2005, 206, 1178–1182. [Google Scholar] [CrossRef]
- Okamura, H.; Kaneko, T.; Shirai, M. Synthesis of Thiophene Oligomers by Photo-irradiation. J. Photopolym. Sci. Technol. 2012, 25, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Diaz, V.; Patel, D.; Underwood, R.; Warren, R. Dissolvable conducting polymer supercapacitor for transient electronics. Org. Electron. 2022, 101, 106412. [Google Scholar] [CrossRef]
- Peng, S.; Zhu, P.; Wu, Y.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun conductive polyaniline–polylactic acid composite nanofibers as counter electrodes for rigid and flexible dye-sensitized solar cells. RSC Adv. 2012, 2, 652–657. [Google Scholar] [CrossRef]
- Fontana-Escartin, A.; Puiggali-Jou, A.; Lanzalaco, S.; Bertran, O.; Aleman, C. Manufactured Flexible Electrodes for Dopamine Detection: Integration of Conducting Polymer in 3D-Printed Polylactic Acid. Adv. Eng. Mater. 2021, 23, 2100002. [Google Scholar] [CrossRef]
- Molina, B.G.; Cuesta, S.; Besharatloo, H.; Roa, J.J.; Armelin, E.; Aleman, C. Free-Standing Faradaic Motors Based on Biocompatible Nanoperforated Poly(lactic Acid) Layers and Electropolymerized Poly(3,4-ethylenedioxythiophene). ACS Appl. Mater. Interfaces 2019, 11, 29427–29435. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Guo, B.; Mad, P.X.; Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 2018, 514, 517–527. [Google Scholar] [CrossRef]
- Puiggali-Jou, A.; Ordono, J.; del Valle, L.J.; Perez-Amodio, S.; Engel, E.; Aleman, C. Tuning multilayered polymeric self-standing films for controlled release of L-lactate by electrical stimulation. J. Control Release 2021, 330, 669–683. [Google Scholar] [CrossRef]
- Xiong, F.; Wei, S.; Sheng, H.; Wu, S.; Liu, Z.; Cui, W.; Sun, Y.; Wu, Y.; Li, B.; Xuan, H.; et al. Three-layer core-shell structure of polypyrrole/polydopamine/poly (L-lactide) nanofibers for wound healing application. Int. J. Biol. Macromol. 2022, 222, 1948–1962. [Google Scholar] [CrossRef]
- Wang, Y.; Zappas, A.J., II; Wilson, J.N.; Kim, I.-B.; Solntsev, K.M.; Tolbert, L.M.; Bunz, U.H.F. Optical Spectroscopy of Grafted Poly(p-phenyleneethynylene)s in Water and Water-DMF Mixtures. Macromolecules 2008, 41, 1112–1117. [Google Scholar] [CrossRef]
- Czelusniak, I.; Szymanska-Buzar, T. Synthesis and characterization of polylactide functionalized polyacetylenes. Eur. Polym. J. 2011, 47, 2111–2119. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Geng, Q.; Zhang, S.; Shen, X.; Kakuchi, R.; Misaka, H.; Kakuchi, T.; Satoh, T.; Sakai, R. Synthesis of a novel one-handed helical poly(phenylacetylene) bearing poly(L-lactide) side chains. Eur. Polym. J. 2011, 47, 1923–1930. [Google Scholar] [CrossRef]
- Kang, E.-H.; Lee, I.-H.; Choi, T.-L. Brush Polymers Containing Semiconducting Polyene Backbones: Graft-Through Synthesis via Cyclopolymerization and Conformational Analysis on the Coil-to-Rod Transition. ACS Macro Lett. 2012, 1, 1098–1102. [Google Scholar] [CrossRef]
- Massoumi, B.; Abbasian, M.; Jahanban-Esfahlan, R.; Mohammad-Rezaei, R.; Khalilzadeh, B.; Samadian, H.; Rezaei, A.; Derakhshankhah, H.; Jaymand, M. A novel bio-inspired conductive, biocompatible, and adhesive terpolymer based on polyaniline, polydopamine, and polylactide as scaffolding biomaterial for tissue engineering application. Int. J. Biol. Macromol. 2020, 147, 1174–1184. [Google Scholar] [CrossRef]
- Baez, J.E.; Marcos-Fernandez, A.; Galindo-Iranzo, P. Exploring the effect of alkyl end group on poly(L-lactide) oligo-esters. Synthesis and characterization. J. Polym. Res. 2011, 18, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/quality/spectra/849/305/FNMR007091.pdf (accessed on 29 November 2022).
- Chafran, L.S.; Campos, J.M.C.; Santos, J.S.; Sales, M.J.A.; Dias, S.C.L.; Dias, J.A. Synthesis of poly(lactic acid) by heterogeneous acid catalysis from D., L-lactic acid. J. Polym. Res. 2016, 23, 107. [Google Scholar] [CrossRef]
- Basterretxea, A.; Gabirondo, E.; Jehanno, C.; Zhu, H.; Coulembier, O.; Mecerreyes, D.; Sardon, H. Stereoretention in the Bulk ROP of L-Lactide Guided by a Thermally Stable Organocatalyst. Macromolecules 2021, 54, 6214–6225. [Google Scholar] [CrossRef]
- Rathi, S.; Kalish, J.P.; Coughlin, E.B.; Hsu, S.L. Utilization of oligo(lactic acid) for studies of chain conformation and chain packing in poly(lactic acid). Macromolecules 2011, 44, 3410–3415. [Google Scholar] [CrossRef]
- Nikolic, L.; Ristic, I.; Adnadjevic, B.; Nikolic, V.; Jovanovic, J.; Stankovic, M. Novel Microwave-Assisted Synthesis of Poly(D,L-lactide): The Influence of Monomer/Initiator Molar Ratio on the Product Properties. Sensors 2010, 10, 5063–5073. [Google Scholar] [CrossRef]
- Pohjakallio, M.; Sundholm, G.; Talonen, P. Adsorption and oxidation of thiophene-3-methanol on platinum electrodes studied by electrochemical and IR spectroscopic methods. J. Electroanal. Chem. 1996, 401, 191–200. [Google Scholar] [CrossRef]
- de França, J.O.C.; da Silva Valadares, D.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers 2022, 14, 2317. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Sun, C.; Ni, L.; Xua, W.; Yuan, W.; Zheng, Y.; Yu, C.; Pan, P. Controllable crystallization and lamellar organization in nucleobase-functionalized supramolecular poly(lactic acid)s: Role of poly (lactic acid) stereostructure. Polymer 2021, 232, 124148. [Google Scholar] [CrossRef]
- Xu, K.; Kozluca, A.; Denkbas, E.B.; Piskin, E. Poly (D,L-lactic acid) homopolymers: Synthesis and characterization. Turk. J. Chem. 1996, 20, 43–53. [Google Scholar]
- Ahmed, J.; Zhang, J.-X.; Song, Z.; Varshney, S.K. Thermal properties of polylactides, Effect of molecular mass and nature of lactide isomer. J. Therm. Anal. Calorim. 2009, 95, 957–964. [Google Scholar] [CrossRef]
- Takizawa, K.; Nulwala, H.; Hu, J.; Yoshinaga, K.; Hawker, C.J. Molecularly defined (L)-lactic acid oligomers and polymers: Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 5977–5990. [Google Scholar] [CrossRef]
- Cicogna, F.; Giachi, G.; Rosi, L.; Passaglia, E.; Coiai, S.; Spiniello, R.; Prescimone, F.; Frediani, M. Macromolecular Dyes by Chromophore-Initiated Ring Opening Polymerization of L-Lactide. Polymers 2020, 12, 1979. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Mullen, K.; Yin, M. Self-assemblies of amphiphilic homopolymers: Synthesis, morphology studies and biomedical applications. Chem. Commun. 2015, 51, 11541–11555. [Google Scholar] [CrossRef]
- Costanzo, G.D.; Ribba, L.; Goyanes, S.; Ledesma, S. Enhancement of the optical response in a biodegradable polymer/azo-dye film by the addition of carbon nanotubes. J. Phys. D Appl. Phys. 2014, 47, 135103. [Google Scholar] [CrossRef]
- Becker, R.S.; de Melo, J.S.; Macuanita, A.L.; Elisei, F. Comprehensive Evaluation of the Absorption, Photophysical, Energy Transfer, Structural, and Theoretical Properties of α-Oligothiophenes with One to Seven Rings. J. Phys. Chem. 1996, 100, 18683–18695. [Google Scholar] [CrossRef] [Green Version]
- Thamizhlarasan, A.; Meenarathi, B.; Parthasarathy, V.; Jancirani, A.; Anbarasan, R. Structural, thermal, spectral and sustainable drug release studies of deoxyfluorouridine tagged poly(d,l-Lactide). Polym. Bull. 2022, 79, 245–262. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Li, S.; Lei, D.; Tang, B.Z.; Ye, R. Recent Advances in Clusteroluminescence. Top. Curr. Chem. 2021, 379, 14. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Chu, B.; Zhang, Z.; Xie, Y.; Wang, L.; Sun, J.Z.; Zhang, H.; Zhang, X.-H.; Tang, B.Z. Manipulation of clusteroluminescence in carbonyl-based aliphatic polymers. Aggregate 2022, 3, e278. [Google Scholar] [CrossRef]
- Patias, G.; Choinopoulos, I.; Koinis, S.; Pitsikalis, M. Employing (Half-) Titanocene Complexes as Initiators for the Synthesis of End-Functionalized Polylactides by Coordination Polymerization. J. Polym. Sci., Part A Polym Chem. 2018, 56, 2192–2202. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Zhao, Y.-M.; Wang, F.; Wang, J. Syntheses of poly(lactic acid-co-glycolic acid) serial biodegradable polymer materials via direct melt polycondensation and their characterization. J. Appl. Polym. Sci. 2006, 99, 244–252. [Google Scholar] [CrossRef]
- Luo, S.-H.; Xiao, Y.; Lin, J.-Y.; Chen, Z.-H.; Lin, S.-T.; Wang, Z.-Y. Preparation, characterization and application of maleic anhydride-modified polylactic acid macromonomer based on direct melt polymerization. Mater. Today Chem. 2022, 25, 100986. [Google Scholar] [CrossRef]
- Koo, D.; Du, A.; Palmese, G.R.; Cairncross, R.A. Synthesis and water sorption of standard and end-capped polylactides: The effect of morphology. Polym. Chem. 2012, 3, 718–726. [Google Scholar] [CrossRef]
- Kopinke, F.-D.; Remmler, M.; Mackenzie, K.; Milder, M.; Wachsen, O. Thermal decomposition of biodegradable polyesters-II. Poly(lactic acid). Polym. Degrad. Stab. 1996, 53, 329–342. [Google Scholar] [CrossRef]
- Wojtczak, E.; Kubisa, P.; Bednarek, M. Thermal stability of polylactide with different end-groups depending on the catalyst used for the polymerization. Polym. Degrad. Stab. 2018, 151, 100–104. [Google Scholar] [CrossRef]
- Tran, H.T.; Matsusaki, M.; Akashi, M.; Vu, N.D. Enhanced Thermal Stability of Polylactide by Terminal Conjugation Groups. J. Electron. Mater. 2016, 45, 2388–2394. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Vacche, S.D.; Vitale, A. Photoinduced Processes as a Way to Sustainable Polymers and Innovation in Polymeric Materials. Polymers 2021, 13, 2293. [Google Scholar] [CrossRef]
- Cianga, L.; Yagci, Y. Synthesis and Characterization of Thiophene-Substituted N-Phenyl Maleimide Polymers by Photoinduced Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 995–1004. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Cianga, I.; Yagci, Y.; Takeichi, T. Photoinitiated Cationic Polymerization of Monofunctional Benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3320–3328. [Google Scholar] [CrossRef]
- Tanabe, M.; Manners, I. Photolytic Living Anionic Ring-Opening Polymerization (ROP) of Silicon-Bridged [1]Ferrocenophanes via an Iron-Cyclopentadienyl Bond Cleavage Mechanism. J. Am. Chem. Soc. 2004, 126, 11434–11435. [Google Scholar] [CrossRef]
- Iyoda, T.; Kitano, M.; Shimidzu, T. New method for preparing poly(benzo[c]thiophene) thin films by photopolymerization. J. Chem. Soc. Chem. Commun. 1991, 22, 1618–1619. [Google Scholar] [CrossRef]
- Woods, E.F.; Berl, A.J.; Kalow, J.A. Advances in the Synthesis of π-Conjugated Polymers by Photopolymerization. ChemPhotoChem 2021, 5, 4–11. [Google Scholar] [CrossRef]
- Kaya, K.; Yagci, Y. Contemporary Approaches for Conventional and Light-Mediated Synthesis of Conjugated Heteroaromatic Polymers. Macromol. Chem. Phys. 2021, 222, 2100334. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Piletska, E.V.; Karim, K.; Davis, F.; Higson, S.P.J.; Turner, A.P.F. Photochemical polymerization of thiophene derivatives in aqueous solution. Chem. Commun. 2004, 19, 2222–2223. [Google Scholar] [CrossRef] [Green Version]
- Bozukova, D.; Jerome, R.; Jerome, C. A fast and facile synthetic route toward the preparation of nanoparticles of polythiophene and its derivatives. J. Nanopart. Res. 2013, 15, 1583. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Mechanism of Photoinduced Step Polymerization of Thiophene by Onium Salts: Reactions of Phenyliodinium and Diphenylsulfinium Radical Cations with Thiophene. Macromolecules 2007, 40, 4481–4485. [Google Scholar] [CrossRef]
- Gomes, A.L.; Zakia, M.B.P.; Filho, J.G.; Armelin, E.; Aleman, C.; de Carvalho Campos, J.S. Preparation and characterization of semiconducting polymeric blends. Photochemical synthesis of poly(3-alkylthiophenes) using host microporous matrices of poly(vinylidene fluoride). Polym. Chem. 2012, 3, 1334–1343. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Cianga, L.; Cianga, I. Poly(ethylene glycol)- functionalized Water Self-Dispersible α-Terthiophenes. Rev. Roum. Chim. 2013, 58, 153–160. [Google Scholar]
- Bendrea, A.-D.; Cianga, L.; Hitruc, E.G.; Cianga, I. Synthesis and Characterization of Fluorescent, Nonionic, Water Self-Dispersible Oligothiophenes. Int. J. Polym. Anal. Charact. 2013, 18, 189–198. [Google Scholar] [CrossRef]
- Pathiranage, T.M.S.K.; Dissanayake, D.S.; Niermann, C.N.; Ren, Y.; Biewer, M.C.; Stefan, M.C. Role of Polythiophenes as Electroactive Materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3327–3346. [Google Scholar] [CrossRef] [Green Version]
- Abbott, S. Chemical compatibility of poly(lactic acid): A practical framework using Hansen solubility parameters. In Poly(lactic acid) Synthesis, Structure, Properties, Processing, and Application; Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 83–89. [Google Scholar]
- Adamska, K.; Voelkel, A.; Berlinska, A. The solubility parameter for biomedical polymers-Application of inverse gas chromatography. J. Pharm. Biomed. 2016, 127, 202–206. [Google Scholar] [CrossRef]
- Siemann, U. The solubility parameter of poly(DL-lactic acid). Eur. Polym. J. 1992, 28, 293–297. [Google Scholar] [CrossRef]
- Hansen, C.M.; Smith, A.L. Using Hansen solubility parameters to correlate solubility of C60 fullerene in organic solvents and in polymers. Carbon 2004, 42, 1591–1597. [Google Scholar] [CrossRef]
- Liu, K.; Fang, C.J.; Li, Z.Q.; Young, M. Separation of thiophene/n-heptane mixtures using PEBAX/PVDF-composited membranes via pervaporation. J. Membr. Sci. 2014, 451, 24–31. [Google Scholar] [CrossRef]
- Barton, A.F. Solubility Parameters. Chem. Rev. 1975, 75, 731–753. [Google Scholar] [CrossRef]
- Orwoll, R.A.; Arnold, P.A. Polymer–Solvent Interaction Parameter χ. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G.A. Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- van Dijk, J.A.P.P.; Smit, J.A.M.; Kohn, F.E.; Feijen, J. Characterization of poly(D,L-lactic acid) by gel permeation chromatography. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.; Acosta-Ramírez, A.; Mehrkhodavandi, P.; Dorgan, J.R.; Hatzikiriakos, S.G. Solution and melt viscoelastic properties of controlled microstructure poly(lactide). J. Rheol. 2011, 55, 987–1004. [Google Scholar] [CrossRef]
- Suzuki, Y.; Watanabe, T.; Kosugi, H.; Ueda, K.; Kikuchi, M.; Narumi, A.; Kawaguchi, S. Dilute solution properties of poly(D,L-lactide) by static light scattering, SAXS, and intrinsic viscosity. Polym. J. 2020, 52, 387–396. [Google Scholar] [CrossRef]
- Noble, J.E. Quantification of Protein Concentration Using UV Absorbance and Coomassie Dyes. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 536, Chapter 2; pp. 17–26. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Zhao, B.; Verdi, C.; Liu, W.; Hao, C.; Zhang, J. Shape memory Poly(lactic acid) binary blends with unusual fluorescence. Polymer 2020, 209, 122980. [Google Scholar] [CrossRef]





| Band Position (cm−1) | Assignment | Band Position (cm−1) | Assignment |
|---|---|---|---|
| 3516 | υ(OH) | 1272 | d(C-H) + υ(C-O-C) |
| 3111 | υ(C-H)α+ υ(C-H)b | 1188 | υas(C-O-C) + ras(CH3) |
| 2998 | υas (CH3) | 1133 | ras(CH3) |
| 2943 | υs (CH3) | 1090 | υs(C-O-C) |
| 2880 | υ(CH) | 1046 | υs(C-CH3) |
| 1755 | υ (C=O) | 955 | r(CH3) + υ(C-C) |
| 1617 | υ(C=C)ring | 916 | r(CH3) + υ(C-C) |
| 1457 | das(CH3) | 862 | υas(C-S)ring |
| 1383 | ds(CH3) | 831 | υs(C-S)ring |
| 1366 | d1(C-H) + ds(CH3) | 795 | g(C-H) |
| 1321 | d2(C-H) | 699 | d(C-S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendrea, A.-D.; Cianga, L.; Göen Colak, D.; Constantinescu, D.; Cianga, I. Thiophene End-Functionalized Oligo-(D,L-Lactide) as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis. Polymers 2023, 15, 1094. https://doi.org/10.3390/polym15051094
Bendrea A-D, Cianga L, Göen Colak D, Constantinescu D, Cianga I. Thiophene End-Functionalized Oligo-(D,L-Lactide) as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis. Polymers. 2023; 15(5):1094. https://doi.org/10.3390/polym15051094
Chicago/Turabian StyleBendrea, Anca-Dana, Luminita Cianga, Demet Göen Colak, Doina Constantinescu, and Ioan Cianga. 2023. "Thiophene End-Functionalized Oligo-(D,L-Lactide) as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis" Polymers 15, no. 5: 1094. https://doi.org/10.3390/polym15051094
APA StyleBendrea, A. -D., Cianga, L., Göen Colak, D., Constantinescu, D., & Cianga, I. (2023). Thiophene End-Functionalized Oligo-(D,L-Lactide) as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis. Polymers, 15(5), 1094. https://doi.org/10.3390/polym15051094