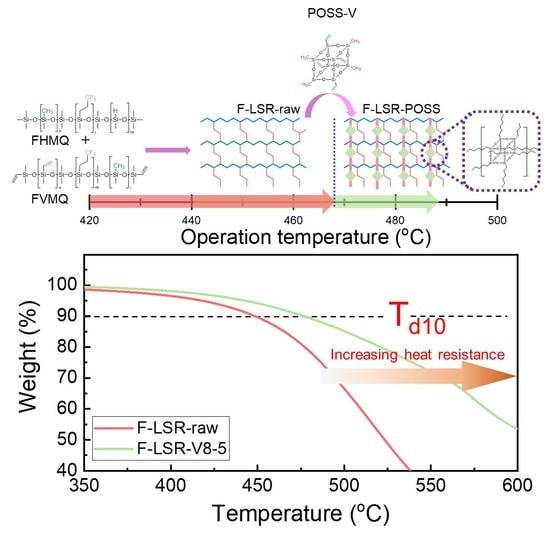
Improvement of Heat Resistance of Fluorosilicone Rubber Employing Vinyl-Functionalized POSS as a Chemical Crosslinking Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. F-Silicone and F-Crosslinker Synthesis
2.3. Synthesis of POSS-Vs
2.4. Preparing F-LSR-POSS
2.5. Characterization
3. Results and Discussion
3.1. Synthesis of F-Silicone and F-Crosslinker
3.2. Synthesis and Characterization of POSS-Vs
3.3. Characterization of F-LSR-POSS
3.4. Mechanical Properties of the F-LSR-POSSs
3.5. Viscoelastic Properties and Crosslinking Density of the F-LSR-POSSs
3.6. Thermal Properties of the F-LSR-POSSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Bai, Y.; Meng, L.; Wang, Y.; Pang, A.; Guo, X.; Xiao, J.; Li, W. A review of poly [(3, 3, 3-trifluoropropyl) methylsiloxane]: Synthesis, properties and applications. Eur. Polym. J. 2022, 163, 110903. [Google Scholar] [CrossRef]
- Roy, R.E.; Indulekha, K.; Vijayalakshmi, K.; Bhuvaneswari, S.; Soumyamol, P.; Rajeev, R. Fluorosilicone polymers with tailored mechanical, acid resistant and adhesive properties: Role of ultrasonication and functionally active single walled carbon nanotubes. Mater. Chem. Phys. 2019, 223, 523–534. [Google Scholar] [CrossRef]
- Zheng, X.; Pang, A.-M.; Wang, Y.; Wang, W.; Bai, Y. Fabrication of UV-curable fluorosilicone coatings with impressive hydrophobicity and solvent resistance. Prog. Org. Coat. 2020, 144, 105633. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Song, J.; Shang, S.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of novel fluorosilicone rubber using imide modified vinyl-containing fluorosilicone resin as cross-linker. J. Polym. Sci. Polym. Chem. 2015, 53, 1769–1776. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, W.; Li, D.; Niu, C.; Feng, S. Preparation and properties of fluorosilicone and fluorosilicone elastomer with various contents of trifluoropropyl groups. e-Polymers 2011, 11, 1. [Google Scholar] [CrossRef]
- Paul, D.R.; Mark, J.E. Fillers for polysiloxane (“silicone”) elastomers. Prog. Polym. Sci. 2010, 35, 893–901. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Appliciations. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Some Novel Polymeric Nanocomposites. Acc. Chem. Res. 2006, 39, 881–888. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, E.J.; Shim, J.H.; Yoon, J.-S. Thermal Stability and Ablation Properties of Silicone Rubber Composites. J. Appl. Polym. Sci. 2008, 110, 1263–1270. [Google Scholar] [CrossRef]
- Verdeho, R.; Saiz-Arroyo, C.; Carretero-Gonzalez, J.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Lopez-Manchado, M.A. Physicla properties of silicone foams filled with carbon nanotubes and functionalized graphene sheets. Eur. Polym. J. 2008, 44, 2790–2797. [Google Scholar] [CrossRef]
- Jiang, M.-J.; Dang, Z.-M.; Xu, H.-P. Enhanced electrical conductivity in chemically modified carbon nanotube/methylvinyl silicone rubber nanocomposite. Eur. Polym. J. 2007, 43, 4924–4930. [Google Scholar] [CrossRef]
- Jia, L.; Du, Z.; Zhang, C.; Li, C.; Li, H. Reinforcement of Polydimethylsiloxane Through Formation of Inorganic-Organic Hybrid Network. Polym. Eng. Sci. 2008, 48, 74–79. [Google Scholar] [CrossRef]
- Inagi, S.; Ogoshi, T.; Miyake, J.; Bertolucci, M.; Fujiwara, T.; Galli, G.; Chiellini, E.; Chujo, Y.; Wynne, K.J. Appearing, Disappearing, and Reappearing Fumed Silica Nanoparticles: Tapping-Mode AFM Evidence in a Condensation Cured Polydimethylsiloxane Hybrid Elastomer. Chem. Mater. 2007, 19, 2141–2143. [Google Scholar] [CrossRef]
- Jindasuwan, S.; Sujaridworakun, P.; Jinawath, S.; Supothina, S. Effect of Heat Treatment Temperature on Surface Topography and Hydrophobicity of Polydimethylsiloxane/Titanium Oxide Hybrid Films. Macromol. Symp. 2008, 264, 90–94. [Google Scholar] [CrossRef]
- Cassagnau, P. Melt rheology of organoclay and fumed silica nanocomposites. Polymer 2008, 49, 2183–2196. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, H.; Xu, J. Cubic Polyhedral Oligomeric Silsesquioxane Based Functional Materials: Synthesis, Assembly, and Applications. Chem. Asian J. 2016, 11, 1322–1337. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedram Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.L.; Krzysztof, P.; Ignazio, B. POSS-Based Polymers. Polymers 2019, 11, 1727. [Google Scholar]
- Ignazio, B. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar]
- Ignazio, B.; Francesco, A.B.; Gianluca, C.; Giulia, C.; Alberta, L.; Antonino, R. Synthesis and Thermal Characterization of New Dumbbell Shaped POSS/PS Nanocomposites: Influence of the Symmetrical Structure of the Nanoparticles on the Dispersion/Aggregation in the Polymer Matrix. Polym. Compos. 2015, 36, 1394–1400. [Google Scholar]
- Ignazio, B.; Abate, L.; Francesco, A.B. Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim. Acta 2017, 655, 117–123. [Google Scholar]
- Joshi, V.; Srividhya, M.; Dubey, M.; Ghosh, A.K.; Saxena, A. Effect of functionalization on dispersion of POSS-silicone rubber nanocomposites. J. Appl. Polym. Sci. 2013, 130, 92–99. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral Oligomeric Silsesquioxanes(POSS)-Based Hybrid Soft Gels: Molecular Design, Material Advantages, and Emerging Applications. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, Y.; Peng, C.; Liu, W.; Peng, Y.; Xu, W. Synthesis and Characterization of Polyhedral Oligomeric Silsesquioxane Hybrid Co-crosslinked Poly(N-isopropylacrylamide-co-dimethylaminoethyl methacrylate) Hydrogels. J. Polym. Sci. 2013, 51, 1494–1504. [Google Scholar] [CrossRef]
- Chen, D.; Yi, S.; Wu, W.; Zhong, Y.; Liao, J.; Huang, C.; Shi, W. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using Vinyl-POSS derivatives as cross linking agents. Polymer 2010, 51, 3867–3878. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.L.; Yang, H.; Zheng, Q. Crosslinking of low density polyethylene with octavinyl polyhedral oligomeric silsesquioxane as the crosslinker. RSC Adv. 2014, 4, 44030. [Google Scholar] [CrossRef]
- Shen, R.; Liu, H. Construction of bimodal silsesquioxane-based porous materials from triphenylphosphine or triphenylphosphine oxide and their size-selective absorption for dye molecules. RSC Adv. 2016, 6, 37731. [Google Scholar] [CrossRef]
- Bing, W.; Minxian, S.; Jie, D.; Zhixiong, H. Polyhedral oligomeric silsesquioxane (POSS)-modified phenolic resin: Synthesis and anti-oxidation properties. e-Polymers 2021, 21, 316–326. [Google Scholar]
- Anna, S.; Anna, K.; Marcin, M.; Tomasz, S.; Krzysztof, S.; Marian, Z. POSS as promoters of self-healing process in silicone composites. Polym. Bull. 2019, 76, 3387–3402. [Google Scholar]
- Indulekha, K.; Behera, P.K.; Rajeev, R.S.; Gouri, C.; Ninan, K.N. Polyfluoroalkyl siloxanes with varying trifluoropropyl conetnt: Synthesis, characterization and solvent resistance studies. J. Fluor. Chem. 2017, 200, 24–32. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Chen, P.; Zhou, X.; Zheng, A.; Guan, Y. Preparation of Fluorosilicone Random Copolymers with Properties Superior to Those of Fluorosilicone/Silcone Polymer Blends. J. Inorg. Organomet. Polym. 2015, 25, 1267–1276. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Shang, S.-B.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of maleated rosin-nodified fluorosilicone resin and its fluorosilicone rubber. J. Appl. Polym. Sci. 2015, 132, 41888. [Google Scholar] [CrossRef]
- Li, Y.-F.S.; Zang, C.-G.; Zhang, Y.-L. Effect of the structure of hydrogen-containing silicone oil on the properties of silicone rubber. Mater. Chem. Phys. 2020, 248, 122734. [Google Scholar] [CrossRef]
- Lampert, F.; Christiansen, A.B.; Din, R.U.; Garcia, Y.G.; Møller, P. Corrsion resistance of AISI 316L coated with an air-cured hydrogen silsesquioxane based spin-on-glass enamel in chloride environment. Corrosion Sci. 2017, 127, 110–119. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M. Effect of Solvent properties on Crystallinity and Morphology of Octavinyl-POSS: A Comparative Study. J. Nanostruct. 2020, 10, 375–391. [Google Scholar]
- Jalarvo, N.; Gourdon, O.; Ehlers, G.; Tyagi, M.; Kumar, S.K.; Dobbs, K.D.; Smalley, R.J.; Guise, W.E.; Ramirez-Cuesta, A.; Wildgruber, C.; et al. Structure and Dynamics of Octamethyl-POSS Nanoparticles. J. Phys. Chem. C 2014, 118, 5579–5592. [Google Scholar] [CrossRef]
- So, J.I.; Shin, D.H.; Kim, J.B.; Jeong, H.W.; Kim, C.H.; Choi, J.; Shim, S.E.; Qian, Y. One-pot synthesis of bifunctional polyhedral oligomeric silsesquioxane: Full spectrum ratio of vinyl groups from 0 to 100%. J. Ind. Eng. Chem. 2022, 113, 502–512. [Google Scholar]
- Waddon, A.J.; Coughlin, E.B. Crystal Structure of Polyhedral Oligomeric Silsequioxane (POSS) Nano-materials: A Study by X-ray Diffraction and Electron Microscopy. Chem. Mater. 2003, 15, 4555–4561. [Google Scholar] [CrossRef]
- Fan, J.; Gong, Z.; Chen, Y. Design of strong fluorosilicone composites via thermodynamic and kinetic regulation of SiO2. Composs. Commun. 2022, 35, 101273. [Google Scholar] [CrossRef]
- Rodriguez, N.; Ruelas, S.; Forien, J.-B.; Dudukovic, N.; DeOtte, J.; Rodriguez, J.; Moran, B.; Lewicki, L.P.; Duoss, E.B.; Oakdale, J.S. 3D Printing of High Viscosity Reinforced Silicone Elastomers. Polymers 2021, 13, 2239. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Zhao, M.; Zhou, H.; Wang, Y.; Ba, M. Effect of PDMS Viscosity and Additive Amount of Curing Agent Solution on the Mechanical Properties of PDMS Fouling Release Coating. J. Phys. Conf. Ser. 2022, 2174, 012036. [Google Scholar] [CrossRef]
- Xu, Q.; Pang, M.; Zhu, L.; Zhang, Y.; Feng, S. Mechanical properties of silicone rubber composed of diverse vinyl content silicone gums blending. Mater. Des. 2010, 31, 4083–4087. [Google Scholar] [CrossRef]
- Qining, K.; Zhipeng, D.; Xuhunag, C. Fluorosilicone resin as a high ultraviolet transmittance optical material. J. Appl. Polym. Sci. 2019, 137, 49102. [Google Scholar]
- Zygo, M.; Lipinska, M.; Lu, Z.; Ilcíková, M.; Bockstaller, M.R.; Mosnacek, J.; Pietrasik, J. New type of montmorillonite compatibilizers and their influence on viscoelastic properties of ethylene propylene diene and methyl vinyl silicone rubbers blends. Appl. Clay Sci. 2019, 183, 105359. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Doi, Y.; Uneyama, T. Entangle Molecular Weight. Nihon Reoeroji Gakkaishi 2020, 48, 177–183. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, J.-J. The Properties of DSC and DMA for Epoxy Nano-and-Micro Mixture Composites. Trans. Elect. Electron. Mater. 2010, 11, 69–72. [Google Scholar] [CrossRef]
- Schweitzer, J.; Merad, S.; Schrodj, G.; Gall, F.B.-L.; Vonna, L. Determination of the Crosslinking Density of a Silicone Elastomer. J. Chem. Educ. 2019, 96, 1472–1478. [Google Scholar] [CrossRef]
- Liu, Z.; Maréchal, P.; Jérôme, R. DMA and DSC investigations of the β transition of poly(vinylidene fluoride). Polymer 1997, 38, 4925–4929. [Google Scholar] [CrossRef]
- Dascalu, M.; Dünki, S.J.; Quinsaat, J.-E.Q.; Ko, Y.S.; Opris, D.M. Synthesis of silicone elastomers containing trifluoropropyl groups and their use in dielectric elastomer transducers. RSC Adv. 2015, 5, 104516–104523. [Google Scholar] [CrossRef]
- Sánchez-Soto, M.; Schiraldi, D.A.; Illescas, S. Study of the morphology and properties of melt-mixed polycarbonate-POSS nanocomposites. Eur. Polym. J. 2009, 45, 341–352. [Google Scholar] [CrossRef]









| Sample | F-LSR (g) | POSS-V | Mol Ratio of Si–H/Si–Vinyl | |
|---|---|---|---|---|
| Type | Amount (phr) | |||
| F-LSR-raw | 300 | / | / | 1 |
| F-LSR-V0-0.5 | POSS-V0 | 0.5 | ||
| F-LSR-V4-0.5 | POSS-V4 | 0.5 | ||
| F-LSR-V6-0.5 | POSS-V6 | 0.5 | ||
| F-LSR-V8-0.5 | POSS-V8 | 0.5 | ||
| F-LSR-V8-1 | POSS-V8 | 1 | ||
| F-LSR-V8-2.5 | POSS-V8 | 2.5 | ||
| F-LSR-V8-5 | POSS-V8 | 5 | ||
| F-LSR-POSSs | Tensile Strength (MPa) | Tear Strength (MPa) | Elongation at Break (%) | Hardness |
|---|---|---|---|---|
| F-LSR-raw | 0.168 | 0.573 | 68.9 | 8 |
| F-LSR-V0-0.5 | 0.223 | 1.002 | 78.5 | 10 |
| F-LSR-V4-0.5 | 0.218 | 0.993 | 71 | 11 |
| F-LSR-V6-0.5 | 0.178 | 1.152 | 67.3 | 11 |
| F-LSR-V8-0.5 | 0.225 | 1.259 | 59.1 | 13 |
| F-LSR-raw | 0.168 | 0.573 | 68.9 | 8 |
| F-LSR-V8-0.5 | 0.225 | 1.259 | 59.1 | 13 |
| F-LSR-V8-1 | 0.255 | 0.748 | 51.2 | 17 |
| F-LSR-V8-2.5 | 0.308 | 0.556 | 34.3 | 25 |
| F-LSR-V8-5 | 0.273 | 0.552 | 19.4 | 29 |
| Sample | Density (g/cm3) | Crosslinking Density ×10−6 (mol/cm3) | Mc (g/mol) |
|---|---|---|---|
| F-LSR-raw | 1.132 | 60.079 | 18,841.870 |
| F-LSR-V0-0.5 | 1.125 | 33.751 | 33,332.627 |
| F-LSR-V4-0.5 | 1.110 | 37.435 | 29,651.351 |
| F-LSR-V6-0.5 | 1.188 | 53.463 | 22,220.857 |
| F-LSR-V8-0.5 | 1.133 | 64.920 | 17,452.329 |
| F-LSR-raw | 1.132 | 60.079 | 18,841.870 |
| F-LSR-V8-0.5 | 1.133 | 64.920 | 17,452.329 |
| F-LSR-V8-1 | 1.118 | 83.476 | 13,393.092 |
| F-LSR-V8-2.5 | 1.151 | 100.983 | 11,397.934 |
| F-LSR-V8-5 | 1.130 | 159.744 | 7073.800 |
| Sample | Tg (DSC) (°C) | Tg (DMA) (°C) | Td10 (°C) | Td30 (°C) | Td50 (°C) | Tdmax (°C) | Residue at 700 °C (wt%) |
|---|---|---|---|---|---|---|---|
| F-LSR-raw | −96.8 | −81.7 | 448.5 | 494.7 | 523.2 | 514.2 | 23.2 |
| F-LSR-V0-0.5 | −96.0 | −79.4 | 454.0 | 500.9 | 530.8 | 530.3 | 19.8 |
| F-LSR-V4-0.5 | −96.1 | −80.9 | 455.4 | 502.5 | 531.6 | 519.1 | 24.5 |
| F-LSR-V6-0.5 | −96.6 | −81.5 | 460.3 | 507.5 | 532.2 | 520.8 | 22.4 |
| F-LSR-V8-0.5 | −96.7 | −81.7 | 461.9 | 511.5 | 537.9 | 529.0 | 25.3 |
| F-LSR-raw | −96.8 | −81.7 | 448.5 | 494.7 | 523.2 | 514.2 | 23.2 |
| F-LSR-V8-0.5 | −96.7 | −81.7 | 461.9 | 511.5 | 537.9 | 529.0 | 25.3 |
| F-LSR-V8-1 | −96.6 | −80.5 | 469.4 | 529.2 | 571.7 | 531.3 | 37.2 |
| F-LSR-V8-2.5 | −96.5- | −81.1 | 473.0 | 548.0 | 587.0 | 571.2 | 40.5 |
| F-LSR-V8-5 | −95.5 | −80.7 | 476.4 | 557.3 | 616.6 | 576.2 | 46.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, J.I.; Lee, C.S.; Kim, B.S.; Jeong, H.W.; Seo, J.S.; Baeck, S.H.; Shim, S.E.; Qian, Y. Improvement of Heat Resistance of Fluorosilicone Rubber Employing Vinyl-Functionalized POSS as a Chemical Crosslinking Agent. Polymers 2023, 15, 1300. https://doi.org/10.3390/polym15051300
So JI, Lee CS, Kim BS, Jeong HW, Seo JS, Baeck SH, Shim SE, Qian Y. Improvement of Heat Resistance of Fluorosilicone Rubber Employing Vinyl-Functionalized POSS as a Chemical Crosslinking Agent. Polymers. 2023; 15(5):1300. https://doi.org/10.3390/polym15051300
Chicago/Turabian StyleSo, Jae Il, Chung Soo Lee, Byeong Seok Kim, Hyeon Woo Jeong, Jin Sung Seo, Sung Hyeon Baeck, Sang Eun Shim, and Yingjie Qian. 2023. "Improvement of Heat Resistance of Fluorosilicone Rubber Employing Vinyl-Functionalized POSS as a Chemical Crosslinking Agent" Polymers 15, no. 5: 1300. https://doi.org/10.3390/polym15051300
APA StyleSo, J. I., Lee, C. S., Kim, B. S., Jeong, H. W., Seo, J. S., Baeck, S. H., Shim, S. E., & Qian, Y. (2023). Improvement of Heat Resistance of Fluorosilicone Rubber Employing Vinyl-Functionalized POSS as a Chemical Crosslinking Agent. Polymers, 15(5), 1300. https://doi.org/10.3390/polym15051300