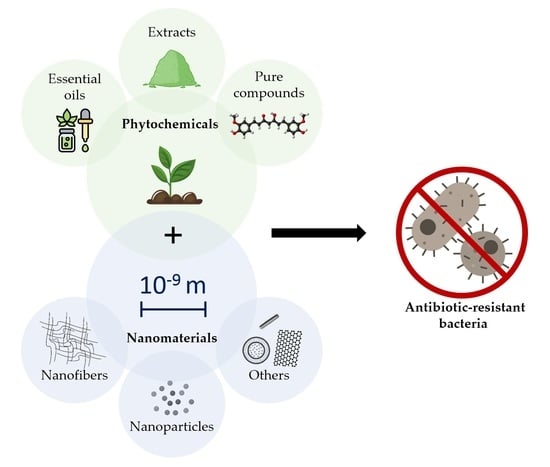
Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review
Abstract
:1. Introduction
2. Study Design
3. Antimicrobial Capacity of Phytochemicals
4. Nanofibers
4.1. Synthesis of Polymeric NFs
4.2. Antibacterial Properties of Polymeric NFs
4.3. Plant-Based NFs against ARB
5. Nanoparticles
5.1. Polymeric NPs
5.1.1. Synthesis of Polymeric NPs
5.1.2. Antibacterial Properties of Polymeric NPs
5.1.3. Plant-Based Polymeric NPs against ARB
5.2. Metal NPs
5.2.1. Synthesis of MNPs
5.2.2. Antibacterial Properties of MNPs
5.2.3. Plant-Based MNPs against ARB
6. Other Plant-Based Nanomaterials
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, J.P.; Ruiz-Alvarez, M.J.; Roson-Calero, N.; Amin, R.; Murugaiyan, J.; van Dongen, M.B.M.; Global, A.M.R.I.A.N. Perspectives on the Ethics of Antibiotic Overuse and on the Implementation of (New) Antibiotics. Infect. Dis. Ther. 2022, 11, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Avershina, E.; Shapovalova, V.; Shipulin, G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021, 12, 2044. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- AlSheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Van vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies-Methods and Approaches to Study the Interaction between Natural Products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The antimicrobial capacity of Cistus salviifolius and Punica granatum plant extracts against clinical pathogens is related to their polyphenolic composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.S.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef] [Green Version]
- Barani, M.; Zeeshan, M.; Kalantar-Neyestanaki, D.; Farooq, M.A.; Rahdar, A.; Jha, N.K.; Sargazi, S.; Gupta, P.K.; Thakur, V.K. Nanomaterials in the Management of Gram-Negative Bacterial Infections. Nanomaterials 2021, 11, 2535. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An Innovative Tool to Release Natural Extracts with Antimicrobial Properties. Pharmaceutics 2021, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Rubio-Camacho, M.; Alarcón, D.; Rodríguez-Cañas, E.; Fernández-Carvajal, A.; Falco, A.; Mallavia, R. L-Menthol-Loadable Electrospun Fibers of PMVEMA Anhydride for Topical Administration. Pharmaceutics 2021, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, J.F.; Barrajón-Catalán, E.; Encinar, A.J.; Rodríguez-Díaz, C.J.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Dimech, G.S.; Soares, L.A.L.; Ferreira, M.A.; de Oliveira, A.G.V.; Carvalho, M.d.C.; Ximenes, E.A. Phytochemical and Antibacterial Investigations of the Extracts and Fractions from the Stem Bark of Hymenaea stigonocarpa Mart. ex Hayne and Effect on Ultrastructure of <i>Staphylococcus aureus</i> Induced by Hydroalcoholic Extract. Sci. World J. 2013, 2013, 862763. [Google Scholar]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef]
- Tiwari, V. Molecular insight into the therapeutic potential of phytoconstituents targeting protein conformation and their expression. Phytomedicine 2019, 52, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Xu, Y.; Zhang, J.; Sui, Z.; Corke, H. Antibacterial Activity and Multi-Targeting Mechanism of Dehydrocorydaline From Corydalis turtschaninovii Bess. Against Listeria monocytogenes. Front. Microbiol. 2022, 12, 3957. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.-o.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Luís, Â.; Oleastro, M.; Domingues, F.; Ferreira, S. Polyphenols as resistance modulators in Arcobacter butzleri. Folia Microbiol. 2019, 64, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Paul, S.M.N. Efficacy of phytochemicals as immunomodulators in managing COVID-19: A comprehensive view. VirusDisease 2021, 32, 435–445. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. New insights in mechanisms of bacterial inactivation by carvacrol. J. Appl. Microbiol. 2013, 114, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Brooker, J.D.; O’Donovan, L.A.; Skene, I.; Clarke, K.; Blackall, L.; Muslera, P. Streptococcus caprinus sp.nov., a tannin-resistant ruminal bacterium from feral goats. Lett. Appl. Microbiol. 1994, 18, 313–318. [Google Scholar] [CrossRef]
- Butler, L.G. Antinutritional Effects of Condensed and Hydrolyzable Tannins. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer US: Boston, MA, USA, 1992; pp. 693–698. [Google Scholar]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial Mechanisms to Overcome Inhibitory Effects of Dietary Tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Becker, S.T.; Podschun, R.; Sivananthan, S.; Springer, I.N.; Russo, P.A.J.; Wiltfang, J.; Fickenscher, H.; Sherry, E. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Cranio-Maxillofac. Surg. 2009, 37, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619. [Google Scholar] [CrossRef]
- Vinayak, M.; Maurya, A. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anti-Cancer Agents Med. Chem. 2019, 19, 1560–1576. [Google Scholar] [CrossRef]
- Torres-Martinez, J.E.; Cornejo Bravo, M.J.; Serrano Medina, A.; Pérez González, L.G.; Villarreal Gómez, J.L. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Martínez-Ortega, L.; Mira, A.; Fernandez-Carvajal, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Development of A New Delivery System Based on Drug-Loadable Electrospun Nanofibers for Psoriasis Treatment. Pharmaceutics 2019, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Nemati, S.; Kim, S.-j.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarrajan, S.; Tan, K.L.; Lim, S.H.; Ramakrishna, S. Electrospun Nanofibers for Air Filtration Applications. Procedia Eng. 2014, 75, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Geng, S.; Pitkänen, O.; Järvinen, T.; Kordas, K.; Oksman, K. Green Carbon Nanofiber Networks for Advanced Energy Storage. ACS Appl. Energy Mater. 2020, 3, 3530–3540. [Google Scholar] [CrossRef]
- Baji, A.; Agarwal, K.; Oopath, S.V. Emerging Developments in the Use of Electrospun Fibers and Membranes for Protective Clothing Applications. Polymers 2020, 12, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari, S.; Mohammadi Ziarani, G.; Badiei, A.; Ajalloueian, F.; Vasseghian, Y. Electrospun composite nanofibers as novel high-performance and visible-light photocatalysts for removal of environmental pollutants: A review. Environ. Res. 2022, 215, 114296. [Google Scholar] [CrossRef]
- Lou, L.; Osemwegie, O.; Ramkumar, S.S. Functional Nanofibers and Their Applications. Ind. Eng. Chem. Res. 2020, 59, 5439–5455. [Google Scholar] [CrossRef]
- Falco, A.; Mallavia, R. Electrospun Nanomaterials: Applications in Food, Environmental Remediation, and Bioengineering. Nanomaterials 2020, 10, 1714. [Google Scholar] [CrossRef]
- Vimala Bharathi, S.K.; Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. 3.43-Recent Trends in Nanocomposite Packaging Materials. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 731–755. [Google Scholar]
- Choi, H.-J.; Kumita, M.; Hayashi, S.; Yuasa, H.; Kamiyama, M.; Seto, T.; Tsai, C.-J.; Otani, Y. Filtration Properties of Nanofiber/Microfiber Mixed Filter and Prediction of its Performance. Aerosol Air Qual. Res. 2017, 17, 1052–1062. [Google Scholar] [CrossRef]
- Deepthi, S. New Perspective of Nano Fibers: Synthesis and Applications. In Nanofibers; Brajesh, K., Ed.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 1. [Google Scholar]
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly(methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 17205. [Google Scholar] [CrossRef] [Green Version]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kumar, N.; Mehrotra, T.; Bisaria, K.; Sinha, S. Chapter 9-Environmental hazards and biodegradation of plastic waste: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–214. [Google Scholar]
- Zeka, K.; Coppa, P.; Corsi, L.; Pajewski, L.A.; Veglio, F.; Continenza, M. In vitro biocompatibility of a new PVP-Hydrogel. Ital. J. Anat. Embryol. 2013, 117, 202. [Google Scholar]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Niu, D.; Hu, C.; Pei, L.Z. Polyethyleneimine-Based Nanocarriers for Gene Delivery. Curr. Pharm. Des. 2015, 21, 6140–6156. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Rehman, W.; Majeed, A.; Mehra, R.; Bhushan, S.; Rani, P.; Saini, K.; Bast, F. Gelatin: A Comprehensive Report Covering its Indispensable Aspects; Nova Science Publishers: New York, NY, USA, 2016; pp. 209–222. [Google Scholar]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as Promising Natural Biodegradable Polymer for Pharmaceutical, Food, and Biomedical Applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Chapter 18-Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; A.R, A., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar]
- Nunes, C.S.; Philipps-Wiemann, P. Chapter 22-Formulation of enzymes. In Enzymes in Human and Animal Nutrition; Nunes, C.S., Kumar, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 429–440. [Google Scholar]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- John, J.V.; McCarthy, A.; Xie, J. Chapter 9-Electrospun nanofiber matrix for tissue repair and regeneration. In Tissue Engineering; Sharma, C.P., Chandy, T., Thomas, V., Thankam, F.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 175–191. [Google Scholar]
- Davoodi, P.; Gill, E.L.; Wang, W.; Shery Huang, Y.Y. Chapter Two-Advances and innovations in electrospinning technology. In Biomedical Applications of Electrospinning and Electrospraying; Kasoju, N., Ye, H., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 45–81. [Google Scholar]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. 11-Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 325–347. [Google Scholar]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Kim, K.; Park, M.-H. On-Demand Drug Delivery Systems Using Nanofibers. Nanomaterials 2021, 11, 3411. [Google Scholar] [CrossRef]
- Mira, A.; Sainz-Urruela, C.; Codina, H.; Jenkins, S.I.; Rodriguez-Diaz, J.C.; Mallavia, R.; Falco, A. Physico-Chemically Distinct Nanomaterials Synthesized from Derivates of a Poly(Anhydride) Diversify the Spectrum of Loadable Antibiotics. Nanomaterials 2020, 10, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, H.B.; Zhang, A.; Eldridge, J.; Demcheva, M.; Tsichilis, P.; Seth, A.; Vournakis, J.; Muise-Helmericks, R.C. Anti-Bacterial Effects of Poly-N-Acetyl-Glucosamine Nanofibers in Cutaneous Wound Healing: Requirement for Akt1. PLoS ONE 2011, 6, e18996. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of Polymeric Nanofibers Loaded with Essential Oils for Biomedical and Food-Packaging Applications. Int. J. Mol. Sci. 2021, 22, 4017. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.W.; Kang, M.J.; Bae, J.H.; Hur, S.B.; Kim, I.-S.; Park, Y.H.; Lee, K.H. Fabrication of Phaeodactylum tricornutum extract-loaded gelatin nanofibrous mats exhibiting antimicrobial activity. Int. J. Biol. Macromol. 2014, 63, 198–204. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Mayandi, V.; Leung, C.M.; Ezhilarasu, H.; Karuppannan, S.K.; Prasannan, P.; Ong, S.T.; Sunderasan, N.; Kaliappan, I.; et al. Core–Shell Structured Antimicrobial Nanofiber Dressings Containing Herbal Extract and Antibiotics Combination for the Prevention of Biofilms and Promotion of Cutaneous Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24356–24369. [Google Scholar] [CrossRef]
- Karuppannan, S.K.; Bushion, J.; Ramalingam, R.; Swaminathan, S.; Arunachalam, K.D.; Kadam, A.A.; Rajagopal, R.; Sathya, R.; Chinnappan, S. Fabrication, characterization and in vitro evaluation of Melia dubia extract infused nanofibers for wound dressing. J. King Saud Univ.-Sci. 2022, 34, 101931. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Lu, W.; Zhang, P. Fabrication of Curcumin@Ag Loaded Core/Shell Nanofiber Membrane and its Synergistic Antibacterial Properties. Front. Chem. 2022, 10, 870666. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-Sabri, A.E.; Almansob, A.; El-Newehy, M.H. Fabrication of Biohybrid Nanofibers by the Green Electrospinning Technique and Their Antibacterial Activity. ACS Omega 2022, 7, 7311–7319. [Google Scholar] [CrossRef] [PubMed]
- Serbezeanu, D.; Bargan, A.; Homocianu, M.; Aflori, M.; Rîmbu, C.M.; Enache, A.A.; Vlad-Bubulac, T. Electrospun Polyvinyl Alcohol Loaded with Phytotherapeutic Agents for Wound Healing Applications. Nanomaterials 2021, 11, 3336. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Shaabani, A. Electrospun biocompatible core/shell polymer-free core structure nanofibers with superior antimicrobial potency against multi drug resistance organisms. Polymer 2016, 101, 151–157. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-Term Effect against Methicillin-Resistant Staphylococcus aureus of Emodin Released from Coaxial Electrospinning Nanofiber Membranes with a Biphasic Profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalingam, N.; Natarajan, T.S.; Rajiv, S. Preparation and characterization of electrospun curcumin loaded poly(2-hydroxyethyl methacrylate) nanofiber—A biomaterial for multidrug resistant organisms. J. Biomed. Mater. Res. Part A 2015, 103, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Kurakula, M.; Koteswara Rao, G.S.N. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of Pharmaceutical and Nutraceutical Active Ingredients Using Electrospinning Processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Stanger, J.; Tucker, N.; Kirwan, K.; Coles, S.; Jacobs, D.; Staiger, M. Effect of Charge Density on the Taylor Cone in Electrospinning. Int. J. Mod. Phys. B 2009, 23, 1956. [Google Scholar] [CrossRef]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rajabi, S.; Shi, C.; Afifirad, G.; Omidi, N.; Kouhsari, E.; Khoshnood, S.; Azizian, K. Antibacterial activity of recently approved antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) strains: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bunachita, S.; Agarwal, A.; Bhamidipati, A.; Patel, U. A Comprehensive Overview of Antibiotic Selection and the Factors Affecting It. Cureus 2021, 13, e13925. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Gumaste, M.R.; Kulkarni, G.A. High mobility and size dependent carrier concentration in CdTe nanoparticles synthesized by single injection hydrothermal method for device applications. Mater. Today Proc. 2021, 45, 3927–3930. [Google Scholar] [CrossRef]
- Neikov, O.D.; Yefimov, N.A. Chapter 9-Nanopowders. In Handbook of Non-Ferrous Metal Powders, 2nd ed.; Neikov, O.D., Naboychenko, S.S., Yefimov, N.A., Eds.; Elsevier: Oxford, UK, 2019; pp. 271–311. [Google Scholar]
- Dorfs, D.; Krahne, R.; Falqui, A.; Manna, L.; Giannini, C.; Zanchet, D. 1.02-Quantum Dots: Synthesis and Characterization. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2011; pp. 17–60. [Google Scholar]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Umerska, A.; Gaucher, C.; Oyarzun-Ampuero, F.; Fries-Raeth, I.; Colin, F.; Villamizar-Sarmiento, M.G.; Maincent, P.; Sapin-Minet, A. Polymeric Nanoparticles for Increasing Oral Bioavailability of Curcumin. Antioxidants 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Feczkó, T. Polymeric nanotherapeutics acting at special regions of body. J. Drug Deliv. Sci. Technol. 2021, 64, 102597. [Google Scholar] [CrossRef]
- Skourtis, D.; Stavroulaki, D.; Athanasiou, V.; Fragouli, P.G.; Iatrou, H. Nanostructured Polymeric, Liposomal and Other Materials to Control the Drug Delivery for Cardiovascular Diseases. Pharmaceutics 2020, 12, 1160. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.; Rai, P.; Pandey, A. Chapter 1-Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Idrees, H.; Zaidi, S.Z.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-u. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Verma, G.; Rajagopalan, M.D.; Valluru, R.; Sridhar, K.A. Chapter 7-Nanoparticles: A Novel Approach to Target Tumors. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–129. [Google Scholar]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Nagavarma, B.V.N.; Yadav, H.; Ayaz, A.; Vasudha, L.; Shivakumar, H. Different techniques for preparation of polymeric nanoparticles- A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- El-Naggar, N.E.-A.; Saber, W.I.A.; Zweil, A.M.; Bashir, S.I. An innovative green synthesis approach of chitosan nanoparticles and their inhibitory activity against phytopathogenic Botrytis cinerea on strawberry leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections. J. Nanobiotechnol. 2020, 18, 156. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Park, J.; Li, C.-H.; Chattopadhyay, A.N.; Nabawy, A.; Landis, R.F.; Gupta, A.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Polymeric Nanoparticles Active against Dual-Species Bacterial Biofilms. Molecules 2021, 26, 4958. [Google Scholar] [CrossRef]
- Conte, C.; Maiolino, S.; Pellosi, D.S.; Miro, A.; Ungaro, F.; Quaglia, F. Polymeric Nanoparticles for Cancer Photodynamic Therapy. In Light-Responsive Nanostructured Systems for Applications in Nanomedicine; Sortino, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 61–112. [Google Scholar]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Fathizadeh, H.; Arkaban, H.; Kalantar-Neyestanaki, D.; Akbarizadeh, M.R.; Turki Jalil, A.; Akhavan-Sigari, R. Recent Advances in Nanotechnology for the Management of Klebsiella pneumoniae-Related Infections. Biosensors 2022, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Kjøniksen, A.-L.; Hiorth, M. Stability of Chitosan Nanoparticles Cross-Linked with Tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Waschinski, C.J.; Herdes, V.; Schueler, F.; Tiller, J.C. Influence of Satellite Groups on Telechelic Antimicrobial Functions of Polyoxazolines. Macromol. Biosci. 2005, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yang, C.; Coady, D.J.; Ong, Z.Y.; Hedrick, J.L.; Yang, Y.-Y. Highly dynamic biodegradable micelles capable of lysing Gram-positive and Gram-negative bacterial membrane. Biomaterials 2012, 33, 1146–1153. [Google Scholar] [CrossRef]
- Haktaniyan, M.; Bradley, M. Polymers showing intrinsic antimicrobial activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef]
- Song, J.; Jung, Y.; Lee, I.; Jang, J. Fabrication of pDMAEMA-coated silica nanoparticles and their enhanced antibacterial activity. J. Colloid Interface Sci. 2013, 407, 205–209. [Google Scholar] [CrossRef]
- Hazra, C.; Kundu, D.; Chatterjee, A.; Chaudhari, A.; Mishra, S. Poly(methyl methacrylate) (core)–biosurfactant (shell) nanoparticles: Size controlled sub-100nm synthesis, characterization, antibacterial activity, cytotoxicity and sustained drug release behavior. Colloids Surf. A Physicochem. Eng. Asp. 2014, 449, 96–113. [Google Scholar] [CrossRef]
- Lalloz, A.; Bolzinger, M.-A.; Faivre, J.; Latreille, P.-L.; Garcia Ac, A.; Rakotovao, C.; Rabanel, J.-M.; Hildgen, P.; Banquy, X.; Briançon, S. Effect of surface chemistry of polymeric nanoparticles on cutaneous penetration of cholecalciferol. Int. J. Pharm. 2018, 553, 120–131. [Google Scholar] [CrossRef]
- Vigliotta, G.; Mella, M.; Rega, D.; Izzo, L. Modulating Antimicrobial Activity by Synthesis: Dendritic Copolymers Based on Nonquaternized 2-(Dimethylamino)ethyl Methacrylate by Cu-Mediated ATRP. Biomacromolecules 2012, 13, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green synthesis of chitosan nanoparticles, optimization, characterization and antibacterial efficacy against multi drug resistant biofilm-forming Acinetobacter baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.S.; Oshiro-Junior, J.A.; Sato, M.R.; Conceição, M.M.; Medeiros, A.C. Polymeric Nanoparticle Associated with Ceftriaxone and Extract of Schinopsis Brasiliensis Engler against Multiresistant Enterobacteria. Pharmaceutics 2020, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Vrouvaki, I.; Koutra, E.; Kornaros, M.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Polymeric Nanoparticles of Pistacia lentiscus var. chia Essential Oil for Cutaneous Applications. Pharmaceutics 2020, 12, 353. [Google Scholar] [CrossRef] [Green Version]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B.; Arshad, M.K.M.; Poopalan, P.; Perumal, V. 2-Nanoparticle synthetic methods: Strength and limitations. In Nanoparticles in Analytical and Medical Devices; Gopinath, S.C.B., Gang, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–43. [Google Scholar]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Marchiol, L. Synthesis of metal nanoparticles in living plants. Ital. J. Agron. 2012, 7, e37. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. JBIC J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Amaro, F.; Morón, Á.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Metallic Nanoparticles—Friends or Foes in the Battle against Antibiotic-Resistant Bacteria? Microorganisms 2021, 9, 364. [Google Scholar] [CrossRef]
- Chuy, G.P.; Muraro, P.C.L.; Viana, A.R.; Pavoski, G.; Espinosa, D.C.R.; Vizzotto, B.S.; da Silva, W.L. Green Nanoarchitectonics of Silver Nanoparticles for Antimicrobial Activity Against Resistant Pathogens. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.S.; Samanta, S.; Dey, S.; Giri, B.; Dash, S.K. Rapid Green Synthesis of Biogenic Silver Nanoparticles Using Cinnamomum tamala Leaf Extract and its Potential Antimicrobial Application Against Clinically Isolated Multidrug-Resistant Bacterial Strains. Biol. Trace Elem. Res. 2020, 198, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 2020, 10, 16781. [Google Scholar] [CrossRef] [PubMed]
- Foroohimanjili, F.; Mirzaie, A.; Hamdi, S.M.M.; Noorbazargan, H.; Hedayati Ch, M.; Dolatabadi, A.; Rezaie, H.; Bishak, F.M. Antibacterial, antibiofilm, and antiquorum sensing activities of phytosynthesized silver nanoparticles fabricated from Mespilus germanica extract against multidrug resistance of Klebsiella pneumoniae clinical strains. J. Basic Microbiol. 2020, 60, 216–230. [Google Scholar] [CrossRef]
- Moorthy, K.; Chang, K.-C.; Wu, W.-J.; Hsu, J.-Y.; Yu, P.-J.; Chiang, C.-K. Systematic Evaluation of Antioxidant Efficiency and Antibacterial Mechanism of Bitter Gourd Extract Stabilized Silver Nanoparticles. Nanomaterials 2021, 11, 2278. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.R.; Khan, R. Green synthesized AgNPs from Periploca hydaspidis Falc. and its biological activities. Microsc. Res. Tech. 2021, 84, 2268–2285. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Ramirez-Rodriguez, P.; Diaz, E.; Wille, H.; Flores-Fernandez, J.M. Biosynthesis of Silver Nanoparticles Using Stenocereus queretaroensis Fruit Peel Extract: Study of Antimicrobial Activity. Materials 2021, 14, 4543. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Rehman, A.A. Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: As an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS ONE 2020, 15, e0234964. [Google Scholar] [CrossRef]
- El-kamel, A.; Ashour, A.; Raafat, D.; El-Goweily, H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: Characterization and antimicrobial properties. Int. J. Nanomed. 2015, 10, 7207. [Google Scholar] [CrossRef] [Green Version]
- Mandhata, C.P.; Sahoo, C.R.; Mahanta, C.S.; Padhy, R.N. Isolation, biosynthesis and antimicrobial activity of gold nanoparticles produced with extracts of Anabaena spiroides. Bioprocess Biosyst. Eng. 2021, 44, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Grinholc, M.; Dena, A.S.A.; El-Sherbiny, I.M.; Megahed, M. Boosting the antibacterial activity of chitosan–gold nanoparticles against antibiotic–resistant bacteria by Punicagranatum L. extract. Carbohydr. Polym. 2021, 256, 117498. [Google Scholar] [CrossRef] [PubMed]
- Ssekatawa, K.; Byarugaba, D.K.; Angwe, M.K.; Wampande, E.M.; Ejobi, F.; Nxumalo, E.; Maaza, M.; Sackey, J.; Kirabira, J.B. Phyto-Mediated Copper Oxide Nanoparticles for Antibacterial, Antioxidant and Photocatalytic Performances. Front. Bioeng. Biotechnol. 2022, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cruz, D.; Vernet-Crua, A.; Mostafavi, E.; González, M.U.; Martínez, L.; Iii, A.A.D.J.; Kusper, M.; Sotelo, E.; Gao, M.; Geoffrion, L.D.; et al. Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects. Nanomaterials 2021, 11, 514. [Google Scholar] [CrossRef]
- Rasha, E.; Monerah, A.; Manal, A.; Rehab, A.; Mohammed, D.; Doaa, E. Biosynthesis of Zinc Oxide Nanoparticles from Acacia nilotica (L.) Extract to Overcome Carbapenem-Resistant Klebsiella Pneumoniae. Molecules 2021, 26, 1919. [Google Scholar] [CrossRef]
- Ahmar Rauf, M.; Oves, M.; Ur Rehman, F.; Rauf Khan, A.; Husain, N. Bougainvillea flower extract mediated zinc oxide’s nanomaterials for antimicrobial and anticancer activity. Biomed. Pharmacother. 2019, 116, 108983. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamar, H.; Rehman, D.S.; Chauhan, D.K.; Tiwari, A.; Upmanyu, V. Green Synthesis, Characterization and Antimicrobial Activity of Copper Oxide Nanomaterial Derived from Momordica charantia. Int. J. Nanomed. 2020, 15, 2541–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindasamy, G.A.; Mydin, R.B.S.M.N.; Sreekantan, S.; Harun, N.H. Compositions and antimicrobial properties of binary ZnO–CuO nanocomposites encapsulated calcium and carbon from Calotropis gigantea targeted for skin pathogens. Sci. Rep. 2021, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Abdul Rahim, R.; Mohammadinejad, R.; Bin Ariff, A. Hydrogel beads bio-nanocomposite based on Kappa-Carrageenan and green synthesized silver nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef] [PubMed]




| Polymer | Phytochemical | Diameter (nm) * | Electrospinning | Antibacterial Activity * | Reference |
|---|---|---|---|---|---|
| Gelatin | Phaeodactylum tricornutuen extract | 700 | Blend | 99.9% inhibition (MRSA) | [77] |
| PCL/gelatin | Gymnema sylvestre LE | 302–340 | Coaxial | ZOI 17.1 mm (MRSA) | [78] |
| PCL/gelatin | Melia dubia extract | 256 | Blend | ZOI 23 mm (MRSA) | [79] |
| PCL/PVP | Curcumin | 880–740 | Coaxial | 37% inhibition (MRSA) | [80] |
| PVA | Myrrh extract | 220 | Blend | ZOI 13.33 mm (DR S. aureus) | [81] |
| Thymus vulgaris extract | 167 | Blend | ZOI 10 mm (MRSA) | [82] | |
| Salvia officinalis folium extract | 143 | ZOI 10 mm (MRSA) | |||
| Hyperici herba extract | 137 | ZOI 10 mm (MRSA) | |||
| PVA/CS | Curcumin | 125 | Blend/Coaxial | 92% inhibition after 6 days (MRSA) | [83] |
| PVP | Emodin | 692 | Coaxial | Growing ZOI (MRSA) | [84] |
| P(HEMA) | Curcumin | 20–110 | Blend | ZOI 17 mm (MRSA), 18 mm (ESBL Escherichia coli) | [85] |
| Silk fibroin/PEO | Manuka honey | 843–2229 | Blend | ZOI 0.7–6.7 mm (MRSA) | [86] |
| Parameters | Effects | References |
|---|---|---|
| Crosslinking | Crosslinked NPs may be more resistant to degradation and may release the antimicrobial agent more slowly. | [119] |
| Micellization | High critical micelle concentration can lead to higher antimicrobial activity due to the greater activity of the polymeric chains as free molecules in solution. | [120] |
| Molecular weight | High molecular weight polymers have shown greater antimicrobial activity against Gram-negative bacteria, due to the entrapment of the polymers by the peptidoglycan layer. | [121] |
| Polymer type and concentration | Some polymers, such as CS or PEI, have intrinsic antimicrobial activities and higher concentration may lead to a greater antimicrobial effect. | [122] |
| Size | Smaller sizes can enhance antimicrobial activity due to internalization to bacterial cells. | [123] |
| Surface area | Larger surface-to-volume NPs provide more active sites for bacterial interaction. | [124] |
| Surface chemistry | Type and density of functional groups in NPs surfaces can affect their antibacterial capacity by influencing their interactions with the bacterial cell surface. | [125] |
| Surface charges | Cationic charges increase antibacterial activity due to interaction with bacterial cell walls. | [126] |
| Polymer | Phytochemical | Synthesis | Diameter (nm) * | Antibacterial Activity * | Reference |
|---|---|---|---|---|---|
| CS | Cardamom EO | Ionic gelation | 50–100 | Growth control for 2 days (MRSA, ESBL E. coli) | [127] |
| CS | Eucalyptus globulus LE | Green synthesis | 7–10 | ZOI of 12–30 (MDR Acinetobacter baumannii) | [128] |
| CS/HPMC | Schinopsis brasiliensis LE/Ceftriaxone | Polyelectrolytic complexation (coacervation) | 150–500 | MIC of 15 µg/mL (ESBL, KPC) | [129] |
| PLA/PVA | Pistacia lentiscus var. chia EO | Solvent evaporation | 240–665 | MIC higher than 3.4 mg/mL (DR Bacillus subtilis sub. spizizenii) | [130] |
| NPs | Phytochemical | Diameter (nm) * | MIC (µg/mL) * | Reference |
|---|---|---|---|---|
| AgNPs | Aloe vera extract | 38.9 | 4.9–9.8 (KPC) | [147] |
| Cinnamomum tamala LE | 10–12 | 12.5 (MDR E. coli), 10 (MDR K. pneumoniae, 12.5 (MDR S. aureus) | [148] | |
| Cotyledon orbiculate LE | 106–137 | 40 (MRSA) | [149] | |
| Flavopunctelia flaventior powder | 69 | 0.156 (MRSA), 0.078 (VRE), 0.019 (MDR Pseudomonas aeruginosa), 0.078 (MDR E. coli) | [150] | |
| Mespilus germanica LE | 17.6 | 6.25–100 (MDR K. pneumoniae) | [151] | |
| Momordica charantia extract | 9.6–16.4 | 4 (CR A. baumannii), 4 (IR A. baumannii) | [152] | |
| Periploca hydaspidis extract | 68.6–114.2 | 10 (MDR K. pneumoniae), 10–20 (MDR S. aureus), 10 (MDR E. coli), 5 (MRSA) | [153] | |
| Stenocereus queretaroensis PE | 60–200 | 0.313 (MRSA) | [154] | |
| Syzygium cumini LE | 10–15 | 8 (MRSA), 20 (VRSA) | [155] | |
| Vaccinium macrocarpon powder | 1.4–8.6 | 18.3–39.5 (MRSA), 9.9–12.7 (MDR P. aeruginosa) | [156] | |
| Xanthoria parietina powder | 145 | 0.078 (MRSA), 0.156 (VRE), 0.039 (MDR P. aeruginosa), 0.156 (MDR E. coli) | [150] | |
| AuNPs | Anabaena spiroides extract | 80 | 25 (MDR Klebsiella oxytoca), 30 (MDR Steptococcus pyogenes), 20 (MRSA) | [157] |
| Punica granatum extract | 39.4 | 15.6 (MRSA) | [158] | |
| CuNPs | Syzygium cumini LE | 30–31 | 14 (MRSA), 16 (VRSA) | [155] |
| CuONPs | Camellia sinensis extract | 61 | 125 (CREC), 125 (CRKP), 30 (MRSA) | [159] |
| Prunus africana BE | 68 | 125 (CREC), 125 (CRKP), 30 (MRSA) | ||
| FeNPs | Syzygium cumini LE | 40–46 | 11 (MRSA), 13 (VRSA) | [155] |
| PdNPs | Padina boryana extract | 8.7 | 125 (MDR S. aureus), 62.5 (MDR E. fergusonii), 62.5 (MDR A. pittii), 62.5 (MDR P. aeruginosa), 62.5 (MDR A. enteropelogenes), 125 (MDR P. mirabilis) | [160] |
| TeNPs | Aloe vera extract | 20–60 | 11.61 (MRSA), 3.53 (MDR E. coli) | [161] |
| ZnONPs | Acacia nilotica extract | 94 | 0.45 (KPC) | [162] |
| Bougainvillea FE | 10–50 | 128 (MRSA), 128 (MREC) | [163] |
| Nanomaterial | Phytochemical | Mean Size (nm) | Synthesis | Antibacterial Activity | Reference |
|---|---|---|---|---|---|
| CuO NRs | Momordica charantia FE | 61.5 × 450 | Green synthesis | ZOI of 28. 66 (MDR S. aureus, S. mutans, C. xerosis), 25.66 (MDR E. coli, P. aeruginosa, S. pyogenes), 27.33 (MDR S. viridans), 23 (MDR S. epidermidis), 31.66 (MDR B. cereus), 24.66 (MDR K. pneumoniae) and 26.33 (MDR P. vulgaris) mm | [168] |
| κ-Carrageenan/AgNPs hydrogel beads | Citrullus colocynthis SE | 25 | Green synthesis/Blending | ZOI of 11 mm (MRSA) | [170] |
| ZnO–CuO nanocomposites | Calotropis gigantea extract | 8.1 × 7.5 | Green synthesis | MIC of 0.16 (MDR P. aeruginosa and MRSA) and 0.63 (MDR K. pneumoniae) | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Falco, A.; Barrajón-Catalán, E.; Mallavia, R. Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review. Polymers 2023, 15, 1392. https://doi.org/10.3390/polym15061392
Díaz-Puertas R, Álvarez-Martínez FJ, Falco A, Barrajón-Catalán E, Mallavia R. Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review. Polymers. 2023; 15(6):1392. https://doi.org/10.3390/polym15061392
Chicago/Turabian StyleDíaz-Puertas, Rocío, Francisco Javier Álvarez-Martínez, Alberto Falco, Enrique Barrajón-Catalán, and Ricardo Mallavia. 2023. "Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review" Polymers 15, no. 6: 1392. https://doi.org/10.3390/polym15061392
APA StyleDíaz-Puertas, R., Álvarez-Martínez, F. J., Falco, A., Barrajón-Catalán, E., & Mallavia, R. (2023). Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review. Polymers, 15(6), 1392. https://doi.org/10.3390/polym15061392