Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane
Abstract
:1. Introduction
2. Materials and Methods
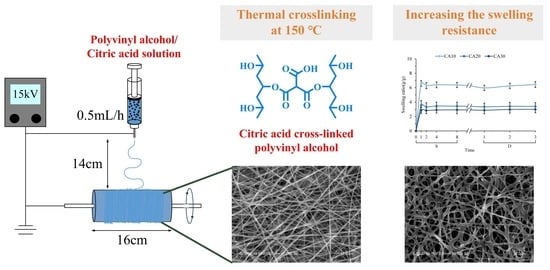
2.1. Materials and Membrane Preparations
2.2. Characterization
2.3. Swelling Characterization
2.4. Mechanical Testing
2.5. Cytotoxicity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Design, Preparation, and Characterization of Electrospun Fibers
3.2. Tensile Strength of Membranes
3.3. FTIR Spectra
3.4. Swelling Resistance of Fibrous Membranes
3.5. In Vitro Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking electrospun poly (vinyl) alcohol fibers with citric acid to impart aqueous stability for medical applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- Hammonds, R.L.; Gazzola, W.H.; Benson, R.S. Physical and thermal characterization of polylactic acid meltblown nonwovens. J. Appl. Polym. Sci. 2014, 131, 40593. [Google Scholar] [CrossRef]
- Parikh, K.S.; Pitha, I.; Hanes, J. Partially Degradable Stents for Controlled Reduction of Intraocular Pressure. U.S. Patent 20190046696A1, 13 March 2019. Available online: https://patents.google.com/patent/US20190046696A1/en (accessed on 16 June 2019).
- Wang, S.; Zhang, Y.; Wang, W.; Li, G.; Ma, X.; Li, X.; Zhang, Z.; Qian, Y. Template-assisted synthesis of porous molybdenum dioxide nanofibers and nanospheres by redox etching method. J. Cryst. Growth 2006, 290, 96–102. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Suzuki, A.; Mikuni, T.; Hasegawa, T. Nylon 66 nanofibers prepared by CO2 laser supersonic drawing. J. Appl. Polym. Sci. 2014, 131, 40015. [Google Scholar] [CrossRef]
- Ichimori, T.; Mizuma, K.; Uchida, T.; Yamazaki, S.; Kimura, K. Morphological diversity and nanofiber networks of poly (p-oxybenzoyl) generated by phase separation during copolymerization. J. Appl. Polym. Sci. 2013, 128, 1282–1290. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Mirata, S.; Sionkowska, A.; Vicini, S.; Alloisio, M.; Castellano, M. Effect of crosslinking type on the physical-chemical properties and biocompatibility of chitosan-based electrospun membranes. Polymers 2021, 13, 831. [Google Scholar] [CrossRef]
- Zárate, I.A.; Aguilar-Bolados, H.; Yazdani-Pedram, M.; Pizarro, G.d.C.; Neira-Carrillo, A. In Vitro hyperthermia evaluation of electrospun polymer composite fibers loaded with reduced graphene oxide. Polymers 2020, 12, 2663. [Google Scholar] [CrossRef]
- Dodero, A.; Brunengo, E.; Castellano, M.; Vicini, S. Investigation of the mechanical and dynamic-mechanical properties of electrospun polyvinylpyrrolidone membranes: A design of experiment approach. Polymers 2020, 12, 1524. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, K.; Zheng, X. Electrospinning and crosslinking of COL/PVA nanofiber-microsphere containing salicylic acid for drug delivery. J. Bionic Eng. 2016, 13, 143–149. [Google Scholar] [CrossRef]
- Cumming, M.H.; Leonard, A.R.; LeCorre-Bordes, D.S.; Hofman, K. Intra-fibrillar citric acid crosslinking of marine collagen electrospun nanofibres. Int. J. Biol. Macromol. 2018, 114, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Czibulya, Z.; Csík, A.; Tóth, F.; Pál, P.; Csarnovics, I.; Zelkó, R.; Hegedűs, C. The effect of the PVA/chitosan/citric acid ratio on the hydrophilicity of electrospun nanofiber meshes. Polymers 2021, 13, 3557. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical properties of electrospun fibers—A critical review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Crețu, B.-E.-B.; Nita, L.E.; Șerban, A.-M.; Rusu, A.G.; Doroftei, F.; Chiriac, A.P. New Cryogels Based on Poly (vinyl alcohol) and a Copolymacrolactone System: I-Synthesis and Characterization. Nanomaterials 2022, 12, 2420. [Google Scholar] [CrossRef]
- Diez, B.; Homer, W.J.A.; Leslie, L.J.; Kyriakou, G.; Rosal, R.; Topham, P.D.; Theodosiou, E. Chemically cross-linked poly (vinyl alcohol) electrospun fibrous mats as wound dressing materials. J. Chem. Technol. Biotechnol. 2022, 97, 620–632. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chen, W.-C.; Haung, S.-M.; Liu, S.-M.; Lin, C.-L. Effects of hinokitiol and dicalcium phosphate on the osteoconduction and antibacterial activity of gelatin-hyaluronic acid crosslinked hydrogel membrane in vitro. Pharmaceuticals 2021, 14, 802. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B 2020, 188, 110757. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Birck, C.; Degoutin, S.; Maton, M.; Neut, C.; Bria, M.; Moreau, M.; Fricoteaux, F.; Miri, V.; Bacquet, M. Antimicrobial citric acid/poly(vinyl alcohol) crosslinked films: Effect of cyclodextrin and sodium benzoate on the antimicrobial activity. LWT-Food Sci. Technol. 2016, 68, 27–35. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.-L.; Nyström, B. Characterization of the chemical degradation of hyaluronic acid during chemical gelation in the presence of different cross-linker agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef]
- Uranga, J.; Leceta, I.; Etxabide, A.; Guerrero, P.; de la Caba, K. Cross-linking of fish gelatins to develop sustainable films with enhanced properties. Eur. Polym. J. 2016, 78, 82–90. [Google Scholar] [CrossRef]
- Shafagh, N.; Sabzi, M.; Afshari, M.J. Development of pH-sensitive and antibacterial gelatin/citric acid/Ag nanocomposite hydrogels with potential for biomedical applications. J. Polym. Res 2018, 25, 259. [Google Scholar] [CrossRef]
- Truong, Y.B.; Choi, J.; Mardel, J.; Gao, Y.; Maisch, S.; Musameh, M.; Kyratzis, I.L. Functional Cross-Linked Electrospun Polyvinyl Alcohol Membranes and Their Potential Applications. Macromol. Mater. Eng. 2017, 302, 1700024. [Google Scholar] [CrossRef] [Green Version]
- Khoswanto, C.; Arijani, E.; Soesilawati, P. Cytotoxicity test of 40, 50 and 60% citric acid as dentin conditioner by using MTT assay on culture cell line. Dent. J. (Maj. Kedokt. Gigi) 2008, 41, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Uppaluri, R.; Das, C. Compositional synergy of poly-vinyl alcohol, starch, glycerol and citric acid concentrations during wound dressing films fabrication. Int. J. Biol. Macromol. 2020, 146, 70–79. [Google Scholar] [CrossRef]
- Yoon, S.D.; Chough, S.H.; Park, H.R. Preparation of resistant starch/poly (vinyl alcohol) blend films with added plasticizer and crosslinking agents. J. Appl. Polym. Sci. 2007, 106, 2485–2493. [Google Scholar] [CrossRef]
- Liu, S.-M.; Chen, W.-C.; Huang, S.-M.; Chen, J.-C.; Lin, C.-L. Characterization of electrospun fibers and electrospray vancomycin-containing beads through the interstitial or lamellar separation of bead composite fiber membranes to evaluate their biomedical application in vitro. J. Ind. Text. 2022, 52, 15280837221139326. [Google Scholar] [CrossRef]
- Chen, W.-C.; Huang, B.-Y.; Huang, S.-M.; Liu, S.-M.; Chang, K.-C.; Ko, C.-L.; Lin, C.-L. In vitro evaluation of electrospun polyvinylidene fluoride hybrid nanoparticles as direct piezoelectric membranes for guided bone regeneration. Biomater. Adv. 2023, 144, 213228. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Hu, H.; Deng, Z.; Pang, L.; Jiang, H.; Wang, D.; Li, J.; Liu, Z.; Wang, H.; Zeng, X. Novel bioactive glass cross-linked PVA hydrogel with enhanced chondrogenesis properties and application in mice chondrocytes for cartilage repair. J. Non-Cryst. Solids 2020, 529, 119594. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Liu, K.; Wu, X.; Dai, H. Citric acid cross-linked chitosan for inhibiting oxidative stress after nerve injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked poly (vinyl alcohol): Structureal, optical and mechanical properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]
- Thiebaud, S.; Aburto, J.; Alric, I.; Borredon, E.; Bikiaris, D.; Prinos, J.; Panayiotou, C. Properties of fatty-acid esters of starch and their blends with LDPE. J. Appl. Polym. Sci. 1997, 65, 705–721. [Google Scholar] [CrossRef]
- Weis, C.; Odermatt, E.K.; Kressler, J.; Funke, Z.; Wehner, T.; Freytag, D. Poly (vinyl alcohol) membranes for adhesion prevention. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70, 191–202. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and characterization of collagen/PVA dual-layer membranes for periodontal bone regeneration. Front. Bioeng. Biotechnol. 2021, 9, 630977. [Google Scholar] [CrossRef]
- Huang, B.; Wu, C.; Hu, Y.; Rao, L.; Yang, M.; Zhao, M.; Chen, H.; Li, Y. Osmanthus-Loaded PVP/PVA Hydrogel Inhibits the Proliferation and Migration of Oral Squamous Cell Carcinoma Cells CAL-27. Polymers 2022, 14, 5399. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, K.; Sui, B.; Boccaccini, A.R.; Sun, J. In vitro evaluation of poly (vinyl alcohol)/collagen blended hydrogels for regulating human periodontal ligament fibroblasts and gingival fibroblasts. Int. J. Biol. Macromol. 2020, 163, 1938–1946. [Google Scholar] [CrossRef]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. Part B Appl. Biomater 2006, 78, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Souriyan-Reyhani pour, H.; Khajavi, R.; Yazdanshenas, M.E.; Zahedi, P.; Mirjalili, M. Cellulose acetate/poly (vinyl alcohol) hybrid fibrous mat containing tetracycline hydrochloride and phenytoin sodium: Morphology, drug release, antibacterial, and cell culture studies. J. Bioact. Compat. Polym. 2018, 33, 597–611. [Google Scholar] [CrossRef]
- García-Hernández, A.B.; Morales-Sánchez, E.; Berdeja-Martínez, B.M.; Escamilla-García, M.; Salgado-Cruz, M.P.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Vega-Cuellar, M.A.; Calderón-Domínguez, G. PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization. Polymers 2022, 14, 1981. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Liu, S.-M.; Tseng, H.-Y.; Chen, W.-C. Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane. Polymers 2023, 15, 1738. https://doi.org/10.3390/polym15071738
Huang S-M, Liu S-M, Tseng H-Y, Chen W-C. Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane. Polymers. 2023; 15(7):1738. https://doi.org/10.3390/polym15071738
Chicago/Turabian StyleHuang, Ssu-Meng, Shih-Ming Liu, Hua-Yi Tseng, and Wen-Cheng Chen. 2023. "Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane" Polymers 15, no. 7: 1738. https://doi.org/10.3390/polym15071738
APA StyleHuang, S. -M., Liu, S. -M., Tseng, H. -Y., & Chen, W. -C. (2023). Effect of Citric Acid on Swelling Resistance and Physicochemical Properties of Post-Crosslinked Electrospun Polyvinyl Alcohol Fibrous Membrane. Polymers, 15(7), 1738. https://doi.org/10.3390/polym15071738