Simultaneously Enhancing the Flame Retardancy, Water Resistance, and Mechanical Properties of Flame-Retardant Polypropylene via a Linear Vinyl Polysiloxane-Coated Ammonium Polyphosphate
Abstract
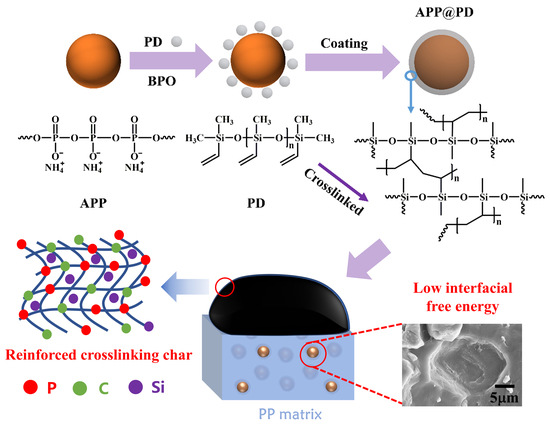
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PD
2.3. Preparation of APP@PD
2.4. Preparation of the Flame-Retardant PP Composites
2.5. Characterization
3. Results and Discussion
3.1. Characterization of PD and APP@PD
3.2. Flame Retardancy of the PP Composites
3.3. Thermal Stability of the PP Composites and Analysis of Their Char Residues
3.4. Immigration of Additives during the Water Immersion Test
3.5. Mechanical Performance of the PP Composites
3.6. Interfacial Interaction between APP and PP before and after the Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Kumar Kundu, C.; Li, Z.; Li, X.; Zhang, Z. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, L.J.; Liu, Y.F.; Qiao, J.J.; Wang, M.T.; Liu, X.Y.; Li, S.S. Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials. Polymers 2022, 14, 2876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiu, S.L.; Chu, F.K.; Xu, Z.M.; Hu, Y. An integrated intumescent flame retardant of bismaleimide from novel maleimide-functionalized triazine-rich polyphosphazene microspheres. Chem. Eng. J. 2022, 450, 138083. [Google Scholar] [CrossRef]
- Qin, Y.L.; Li, M.C.; Huang, T.K.; Shen, C.H.; Gao, S.J. A study on the modification of polypropylene by a star-shaped intumescent flame retardant containing phosphorus and nitrogen. Polym. Degrad. Stab. 2022, 195, 109801. [Google Scholar] [CrossRef]
- Wang, N.; Mi, L.; Wu, Y.X.; Zhang, J.; Fang, Q.H. Double-layered co-microencapsulated ammonium polyphosphate and mesoporous MCM-41 in intumescent flame-retardant natural rubber composites. J. Therm. Anal. Calorim. 2014, 115, 1173–1181. [Google Scholar] [CrossRef]
- Yan, H.W.; Wei, J.L.; Yin, B.; Yang, M.B. Effect of the surface modification of ammonium polyphosphate on the structure and property of melamine-formaldehyde resin microencapsulated ammonium polyphosphate and polypropylene flame retardant composites. Polym. Bull. 2015, 72, 2725–2737. [Google Scholar] [CrossRef]
- Liu, Z.T.; Dai, M.Q.; Hu, Q.H.; Liu, S.; Gao, X.; Ren, F.; Zhang, Q. Effect of microencapsulated ammonium polyphosphate on the durability and fire resistance of waterborne intumescent fire-retardant coatings. J. Coat. Technol. Res. 2019, 16, 135–145. [Google Scholar] [CrossRef]
- Huang, Z.; Ruan, B.; Wu, J.; Ma, N.; Jiang, T.; Tsai, F.-C. High-efficiency ammonium polyphosphate intumescent encapsulated polypropylene flame retardant. J. Appl. Polym. Sci. 2020, 138, 50413. [Google Scholar] [CrossRef]
- Wu, K.; Song, L.; Wang, Z.Z.; Hu, Y. Preparation and characterization of double shell microencapsulated ammonium polyphosphate and its flame retardance in polypropylene. J. Polym. Res. 2009, 16, 283–294. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, H.H.; Yang, Y.D.; Chen, D.P.; Liu, C.L.; Zhang, P.; Zhou, L.; Huang, X.J.; Zhang, H.; Liu, X.Y. Preparation of melamine-formaldehyde resin-microencapsulated ammonium polyphosphate and its application in flame retardant rigid polyurethane foam composites. J. Polym. Res. 2020, 27, 375. [Google Scholar] [CrossRef]
- Decsov, K.; Bocz, K.; Szolnoki, B.; Bourbigot, S.; Fontaine, G.; Vadas, D.; Marosi, G. Development of Bioepoxy Resin Microencapsulated Ammonium-Polyphosphate for Flame Retardancy of Polylactic Acid. Molecules 2019, 24, 4123. [Google Scholar] [CrossRef]
- Gao, W.; Wang, S.; Ma, H.; Wang, Y.; Meng, F. Combined Situ Polymerization and Thermal Cross-Linking Technique for the Preparation of Ammonium Polyphosphate Microcapsules with Composite Shell. J. Phys. Chem. C 2015, 119, 28999–29005. [Google Scholar] [CrossRef]
- Gao, W.; Wang, S.; Meng, F.; Wang, Y.; Ma, H. Microencapsulated ammonium polyphosphate with boron-modified phenolic resin. J. Appl. Polym. Sci. 2016, 133, 43720. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Li, D.-F.; Fu, T.; He, L.; Wang, X.-L.; Wang, Y.-Z. Biomimetic construction peanut-leaf structure on ammonium polyphosphate surface: Improving its compatibility with poly(lactic acid) and flame-retardant efficiency simultaneously. Chem. Eng. J. 2021, 412, 128737. [Google Scholar] [CrossRef]
- Yu, S.; Xiao, S.; Zhao, Z.; Huo, X.; Wei, J. Microencapsulated ammonium polyphosphate by polyurethane with segment of dipentaerythritol and its application in flame retardant polypropylene. Chin. J. Chem. Eng. 2019, 27, 1735–1743. [Google Scholar] [CrossRef]
- Wang, B.B.; Tang, Q.B.; Hong, N.N.; Song, L.; Wang, L.; Shi, Y.Q.; Hu, Y. Effect of Cellulose Acetate Butyrate Microencapsulated Ammonium Polyphosphate on the Flame Retardancy, Mechanical, Electrical, and Thermal Properties of Intumescent Flame-Retardant Ethylene-Vinyl Acetate Copolymer/Microencapsulated Ammonium Polyphosphate/Polyamide-6 Blends. ACS Appl. Mater. Interfaces 2011, 3, 3754–3761. [Google Scholar]
- Zheng, Z.H.; Qiang, L.H.; Yang, T.; Wang, B.N.; Cui, X.J.; Wang, H.Y. Preparation of microencapsulated ammonium polyphosphate with carbon source- and blowing agent-containing shell and its flame retardance in polypropylene. J. Polym. Res. 2014, 21, 443. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Liu, Y.L.; Liao, H.H. Flame-retardant epoxy resins: An approach from organic-inorganic hybrid nanocomposites. J. Polym. Sci. Pol. Chem. 2001, 39, 986–996. [Google Scholar] [CrossRef]
- Deng, C.L.; Du, S.L.; Zhao, J.; Shen, Z.Q.; Deng, C.; Wang, Y.Z. An intumescent flame retardant polypropylene system with simultaneously improved flame retardancy and water resistance. Polym. Degrad. Stab. 2014, 108, 97–107. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Lu, X.; Yang, H.Y.; Xin, Z. Vinyl polysiloxane microencapsulated ammonium polyphosphate and its application in flame retardant polypropylene. J. Polym. Res. 2018, 25, 7. [Google Scholar] [CrossRef]
- Yang, W.; Dong, X.; Nie, S.B.; Yang, J.N.; Zhang, X.F.; Wu, X.Q.; Fang, C.Y.; Su, H.L. Flame-retardant and thermal properties of highly efficient water-resistant intumescent flame-retardant polypropylene composites. J. Therm. Anal. Calorim. 2022, 147, 7323–7336. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; Liu, M.; Li, C.; Meng, L.; Hou, S. Modified Ammonium Polyphosphate and Its Application in Polypropylene Resins. Coatings 2022, 12, 1738. [Google Scholar] [CrossRef]
- Liu, J.C.; Xu, M.J.; Lai, T.; Li, B. Effect of Surface-Modified Ammonium Polyphosphate with KH550 and Silicon Resin on the Flame Retardancy, Water Resistance, Mechanical and Thermal Properties of Intumescent Flame Retardant Polypropylene. Ind. Eng. Chem. Res. 2015, 54, 9733–9741. [Google Scholar] [CrossRef]
- Ran, G.W.; Liu, X.D.; Guo, J.; Sun, J.; Li, H.F.; Gu, X.Y.; Zhang, S. Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos. Pt. B-Eng. 2019, 173, 106772. [Google Scholar] [CrossRef]
- Hoang, D.T.; Schorr, D.; Landry, V.; Blanchet, P.; Vanslambrouck, S.; Dagenais, C. Preparation and characterisation of flame retardant encapsulated with functionalised silica-bosed shell. J. Microencapsul. 2018, 35, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jin, Q.; Zhang, N.; Guo, X.; Yan, H. Preparation of a novel polysiloxane and its synergistic effect with ammonium polyphosphate on the flame retardancy of polypropylene. Polym. Degrad. Stab. 2018, 150, 73–85. [Google Scholar] [CrossRef]
- Ma, Z.-L.; Lu, G.-Y. Effect of alkyl silicone oil on the compatibility of polypropylene/microencapsulated ammonium polyphosphate composites. J. Appl. Polym. Sci. 2011, 121, 1176–1182. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiao, C.M. Synergistic effects of hydroxy silicone oil on intumescent flame retardant polypropylene system. Fire Saf. J. 2009, 44, 1010–1014. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.; Feng, H.; Jin, S.; Hao, J. Toughening Effect and Flame-Retardant Behaviors of Phosphaphenanthrene/Phenylsiloxane Bigroup Macromolecules in Epoxy Thermoset. Macromolecules 2018, 51, 9992–10002. [Google Scholar] [CrossRef]
- Marosi, G.; Tohl, A.; Bertalan, G.; Anna, P.; Maatoug, M.A.; Ravadits, I.; Bertoti, I.; Toth, A. Modified interfaces in multicomponent polypropylene fibers. Compos. Pt. A-Appl. Sci. Manuf. 1998, 29, 1305–1311. [Google Scholar] [CrossRef]
- Chen, X.L.; Hu, Y.; Jiao, C.M.; Song, L. Preparation and thermal properties of a novel flame-retardant coating. Polym. Degrad. Stab. 2007, 92, 1141–1150. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar]
- Gao, S.L.; Li, B.; Bai, P.; Zhang, S.Q. Effect of polysiloxane and silane-modified SiO2 on a novel intumescent flame retardant polypropylene system. Polym. Adv. Technol. 2011, 22, 2609–2616. [Google Scholar] [CrossRef]
- Crowe, C.D.; Hendrickson-Stives, A.K.; Kuhn, S.L.; Jackson, J.B.; Keating, C.D. Designing and 3D Printing an Improved Method of Measuring Contact Angle in the Middle School Classroom. J. Chem. Educ. 2021, 98, 1997–2004. [Google Scholar] [CrossRef]
- Kabir, H.; Garg, N. Machine learning enabled orthogonal camera goniometry for accurate and robust contact angle measurements. Sci. Rep. 2023, 13, 1497. [Google Scholar] [CrossRef]
- Pappalardo, S.; Russo, P.; Acierno, D.; Rabe, S.; Schartel, B. The synergistic effect of organically modified sepiolite in intumescent flame retardant polypropylene. Eur. Polym. J. 2016, 76, 196–207. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.J.; Li, B. Synthesis of N-methyl triazine-ethylenediamine copolymer charring foaming agent and its enhancement on flame retardancy and water resistance for polypropylene composites. Polym. Degrad. Stab. 2016, 131, 20–29. [Google Scholar] [CrossRef]
- Shao, Z.B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.J.; Chen, L.; Wang, Y.Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Pötschke, P.; Pegel, S.; Claes, M.; Bonduel, D. A Novel Strategy to Incorporate Carbon Nanotubes into Thermoplastic Matrices. Macromol. Rapid Commun. 2008, 29, 244–251. [Google Scholar] [CrossRef]
- Comyn, J. Contact angles and adhesive bonding. Int. J. Adhes. Adhes. 1992, 12, 145–149. [Google Scholar] [CrossRef]
















| Sample | Untreated | After Water Treatment | |||
|---|---|---|---|---|---|
| LOI (%) | UL-94 | LOI (%) | ΔLOI a | UL-94 | |
| PP | 18.7 ± 0.1 | NR b | 18.7 ± 0.1 | 0 | NR |
| PP/25APP | 21.8 ± 0.3 | NR | 21.2 ± 0.2 | 2.8 | NR |
| PP/25APP@PD | 27.1 ± 0.2 | NR | 26.7 ± 0.2 | 1.5 | NR |
| PP/25(APP/DPER) | 29.6 ± 0.2 | V-0 | 26.0 ± 0.2 | 12.2 | NR |
| PP/25(APP@PD/DPER) | 31.7 ± 0.3 | V-0 | 28.3 ± 0.2 | 10.7 | V-0 |
| PP/19(APP/DPER) | 26.3 ± 0.3 | NR | 24.0 ± 0.3 | 8.7 | NR |
| PP/19(APP@PD/DPER) | 28.3 ± 0.2 | V-0 | 26.4 ± 0.2 | 6.7 | NR |
| Sample | Td,5% (°C) | Tmax (°C) | Residue (wt.%) | ||
|---|---|---|---|---|---|
| 600 °C | 700 °C | 800 °C | |||
| PP | 436 | 491 | 0.1 | 0.1 | 0.1 |
| PP/APP/DPER | 395 | 502 | 9.3 | 7.4 | 5.7 |
| PP/APP@PD/DPER | 397 | 499 | 9.6 | 9.0 | 8.4 |
| Sample | (mJ·m−2) | (mJ·m−2) | (mJ·m−2) | (mJ·m−2) |
|---|---|---|---|---|
| PP | 2.95 | 26.28 | 29.23 | |
| APP | 29.89 | 40.84 | 70.73 | 15.66 |
| APP@PD | 0.02 | 26.50 | 26.52 | 2.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Q.; Bai, J.; Zhang, G.; Zhang, J.; Yang, M. Simultaneously Enhancing the Flame Retardancy, Water Resistance, and Mechanical Properties of Flame-Retardant Polypropylene via a Linear Vinyl Polysiloxane-Coated Ammonium Polyphosphate. Polymers 2023, 15, 2074. https://doi.org/10.3390/polym15092074
Ke Q, Bai J, Zhang G, Zhang J, Yang M. Simultaneously Enhancing the Flame Retardancy, Water Resistance, and Mechanical Properties of Flame-Retardant Polypropylene via a Linear Vinyl Polysiloxane-Coated Ammonium Polyphosphate. Polymers. 2023; 15(9):2074. https://doi.org/10.3390/polym15092074
Chicago/Turabian StyleKe, Qining, Junchen Bai, Ge Zhang, Jiacheng Zhang, and Mingshu Yang. 2023. "Simultaneously Enhancing the Flame Retardancy, Water Resistance, and Mechanical Properties of Flame-Retardant Polypropylene via a Linear Vinyl Polysiloxane-Coated Ammonium Polyphosphate" Polymers 15, no. 9: 2074. https://doi.org/10.3390/polym15092074
APA StyleKe, Q., Bai, J., Zhang, G., Zhang, J., & Yang, M. (2023). Simultaneously Enhancing the Flame Retardancy, Water Resistance, and Mechanical Properties of Flame-Retardant Polypropylene via a Linear Vinyl Polysiloxane-Coated Ammonium Polyphosphate. Polymers, 15(9), 2074. https://doi.org/10.3390/polym15092074