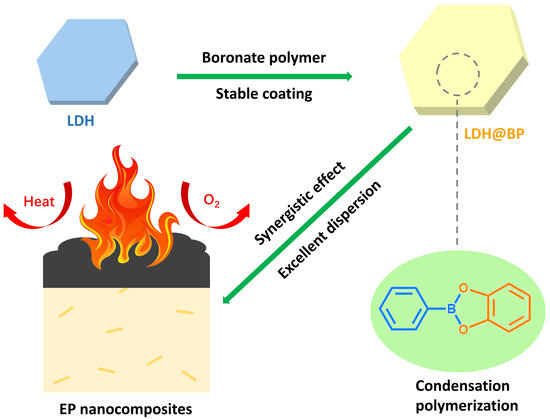
LDH@Boronate Polymer Core–Shell Nanoparticles: Nanostructure Design for Synergistically Enhancing the Flame Retardancy of Epoxy Resin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Monomer Synthesis
2.3. Preparation of MgAl-LDH
2.4. Preparation of LDH@BP Nanoparticles
2.5. Preparation of EP/LDH@BP Nanocomposites
2.6. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of LDH@BP Nanoparticles
3.2. Dispersion and Compatibilities
3.3. Thermal Stability
3.4. Flame Retardancy of EP Nanocomposites
3.5. Flame-Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, M.; Mu, X.; Cai, W.; Wang, X.; Ye, D.; Xi, J.; Hu, Y.; Xing, W. Covalent organic framework with Cu-containing compounds for enhancing flame retardancy and smoke suppression effects on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106900. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Cho, J.; Yeo, H.; Lee, B.W.; Moon, B.J.; Ha, Y.-M.; Jo, Y.R.; Jung, Y.C. Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener. ACS Sustain. Chem. Eng. 2019, 7, 3858–3865. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Li, Z.; Huang, G.; Wu, G. Efficient flame retardancy, good thermal stability, mechanical enhancement, and transparency of DOPO-conjugated structure compound on epoxy resin. Chem. Eng. J. 2022, 450, 138424. [Google Scholar] [CrossRef]
- Mathews, L.D.; Capricho, J.C.; Peerzada, M.; Salim, N.V.; Parameswaranpillai, J.; Hameed, N. Recent progress and multifunctional applications of fire-retardant epoxy resins. Mater. Today Commun. 2022, 33, 104702. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V.; Plasseraud, L.; Boni, G. New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins. ACS Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Zabihi, O.; Li, Q.; Ahmadi, M.; Yadav, R.; Kalali, E.N.; Tanwar, K.; Kiziltas, A.; Blanchard, P.; Wang, D.-Y.; et al. Organophosphorus-Functionalized Zirconium-Based Metal–Organic Framework Nanostructures for Improved Mechanical and Flame Retardant Polymer Nanocomposites. ACS Appl. Nano Mater. 2021, 4, 13027–13040. [Google Scholar] [CrossRef]
- He, W.; Song, P.; Yu, B.; Fang, Z.; Wang, H. Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Prog. Mater. Sci. 2020, 114, 100687. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Gao, S.; Yang, X.; Fan, R.; Zhi, M.; Fu, M. Recent advances in the flame retardancy role of graphene and its derivatives in epoxy resin materials. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106539. [Google Scholar] [CrossRef]
- Bao, Q.; He, R.; Liu, Y.; Wang, Q. Multifunctional boron nitride nanosheets cured epoxy resins with highly thermal conductivity and enhanced flame retardancy for thermal management applications. Compos. Part A Appl. Sci. Manuf. 2023, 164, 107309. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Wang, Z.; He, X.; Wang, C.; Lin, H.; Deng, Y. MOFs-derived self-sacrificing template strategy to double-shelled metal oxides nanocages as hierarchical interfacial catalyst for suppressing smoke and toxic gases releases of epoxy resin. Chem. Eng. J. 2021, 432, 134328. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, W.; Lu, H.; Song, K.; Geng, Z.; Ye, X.; Pan, Y.-T.; Zhang, W.; Yang, R. Multielement Flame-Retardant System Constructed with Metal POSS–Organic Frameworks for Epoxy Resin. ACS Appl. Mater. Interfaces 2022, 14, 49326–49337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, J.; Zhang, W.; Pan, Y.-T.; Wang, D.-Y.; Yang, R. Synthesis of a novel dual layered double hydroxide hybrid nanomaterial and its application in epoxy nanocomposites. Chem. Eng. J. 2020, 381, 122777. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, H.; Tzounis, L.; Santagiuliana, G.; Bilotti, E.; Papageorgiou, D.G. Multifunctional epoxy nanocomposites reinforced by two-dimensional materials: A review. Carbon 2021, 185, 57–81. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, K.; Jiao, E.; Chen, W.; Hu, Z.; Xu, C.; Shi, J.; Wang, S.; Tan, Z. Surface functionalization of few-layer black phosphorene and its flame retardancy in epoxy resin. Chem. Eng. J. 2019, 382, 122991. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Liu, T.; Ou, R.; Lin, J.; Puglia, D.; Xu, P.; Wang, Q.; Dong, W.; Du, M.; et al. Design of Intrinsically Flame-Retardant Vanillin-Based Epoxy Resin for Thermal-Conductive Epoxy/Graphene Aerogel Composites. ACS Appl. Mater. Interfaces 2021, 13, 59341–59351. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhu, Q.; Wang, X.; Shao, Z.; Yao, L.; Zeng, H.; Wu, X.; Chen, J.; Huang, K.; Feng, S. Formation and Stabilization of NiOOH by Introducing alpha-FeOOH in LDH: Composite Electrocatalyst for Oxygen Evolution and Urea Oxidation Reactions. Adv. Mater. 2022, 35, 2209338. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Wei, J.-J.; Zeng, G.-M.; Zhang, H.-Q.; Tan, X.-F.; Ma, C.; Li, X.-C.; Li, Z.-H.; Zhang, C. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Hu, J.; Tang, X.; Dai, Q.; Liu, Z.; Zhang, H.; Zheng, A.; Yuan, Z.; Li, X. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Chen, F.; Chen, C.; Hu, Q.; Xiang, B.; Song, T.; Zou, X.; Li, W.; Xiong, B.; Deng, M. Synthesis of CuO@CoNi LDH on Cu foam for high-performance supercapacitors. Chem. Eng. J. 2020, 401, 126145. [Google Scholar] [CrossRef]
- Zhou, X.; Mu, X.; Cai, W.; Wang, J.; Chu, F.; Xu, Z.; Song, L.; Xing, W.; Hu, Y. Design of Hierarchical NiCo-LDH@PZS Hollow Dodecahedron Architecture and Application in High-Performance Epoxy Resin with Excellent Fire Safety. ACS Appl. Mater. Interfaces 2019, 11, 41736–41749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.; He, L.; Guan, Q.; Xu, X.; Miao, R.; Wang, M.; Zhou, H. Investigation on guanidine phosphate modified LDH and its flame-retardant mechanism in cellulosic composites. Appl. Clay Sci. 2022, 228, 106646. [Google Scholar] [CrossRef]
- Li, P.; Dang, L.; Li, Y.; Lan, S.; Zhu, D. Enhanced flame-retardant and mechanical properties of epoxy resin by combination with layered double hydroxide, Mg2B2O5 whisker, and dodecyl dihydrogen phosphate. Mater. Des. 2022, 217, 110608. [Google Scholar] [CrossRef]
- Zhong, C.-Z.; Xu, S.; Liu, Z.-H.; Lu, J.-J.; Yang, Y.-M.; Li, J.-S.; Wang, N.-L.; Wu, K.; Ding, C.-J.; Zeng, H.-Y. Fabrication of hierarchical core–shell carbon microspheres@ layered double hydroxide@ polyphosphazene architecture in flame-retarding polypropylene. Eur. Polym. J. 2022, 177, 111405. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Liu, Y.; Zhang, S.; Wu, Z. Convenient synthesis of one-dimensional a-SEP@LDH via self-assembly towards simultaneously improved fire retardance, mechanical strength and thermal resistance for epoxy resin. Compos. Part B Eng. 2021, 216, 108857. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Zhang, Z.; Wang, Q. Preparation of ammonium polyphosphate and dye co-intercalated LDH/polypropylene composites with enhanced flame retardant and UV resistance properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, X.; Li, H.; Gao, J.; Zeng, X.; Liao, X. Facile fabrication of a novel polyborosiloxane-decorated layered double hydroxide for remarkably reducing fire hazard of silicone rubber. Compos. Part B Eng. 2019, 175, 107068. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Shi, D.; Song, F. Synthesis and application of low-cost layered double hydroxides intercalated by gluconic acid anion for flame retardancy and tensile strength conservation of high filling epoxy resin. J. Colloid Interface Sci. 2021, 594, 791–801. [Google Scholar] [CrossRef]
- Hu, J.; Xu, S.; Wang, H.; Ding, C.-J.; Liu, Z.-H.; Ma, J.-X.; Li, Z.-Z.; Wang, Q.; Zhu, J.-Y.; Qu, N.; et al. Dual Modification of Layered Double Hydroxide by Phosphonitrilic Chloride Trimer and Aniline for Enhancing the Flame Retardancy of Polypropylene. ACS Appl. Polym. Mater. 2022, 4, 4166–4178. [Google Scholar] [CrossRef]
- Huang, S.-C.; Deng, C.; Wang, S.-X.; Wei, W.-C.; Chen, H.; Wang, Y.-Z. Electrostatic action induced interfacial accumulation of layered double hydroxides towards highly efficient flame retardance and mechanical enhancement of thermoplastic polyurethane/ammonium polyphosphate. Polym. Degrad. Stab. 2019, 165, 126–136. [Google Scholar] [CrossRef]
- Zhou, K.; Gong, K.; Gao, F.; Yin, L. Facile strategy to synthesize MXene@LDH nanohybrids for boosting the flame retardancy and smoke suppression properties of epoxy. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106912. [Google Scholar] [CrossRef]
- Xu, Z.P.; Stevenson, G.S.; Lu, C.-Q.; Lu, G.Q.; Bartlett, P.F.; Gray, P.P. Bartlett, and Peter P. Gray. Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 2005, 128, 36–37. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, T.; Mao, J.; Chen, T.; Li, Y.; Li, M.; Xu, Y.; Zeng, B.; Luo, W.; Yu, L.; et al. Predictable Particle Engineering: Programming the Energy Level, Carrier Generation, and Conductivity of Core–Shell Particles. J. Am. Chem. Soc. 2018, 140, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Acharya, H. Selective Removal of Anionic Dyes by Metal–Organic Framework-Anchored CoAl-Layered Double Hydroxide Nanosheets. ACS Appl. Nano Mater. 2021, 4, 12561–12575. [Google Scholar] [CrossRef]
- Li, M.; Hao, X.; Hu, M.; Huang, Y.; Qiu, Y.; Li, L. Synthesis of bio-based flame-retardant epoxy co-curing agent and application in wood surface coating. Prog. Org. Coat. 2022, 167, 106848. [Google Scholar] [CrossRef]
- Xia, L.; Miao, Z.; Dai, J.; Zhu, A.; Xu, H.; Zhong, J.; Chen, Y.; Luo, W.; Xu, Y.; Yuan, C.; et al. Facile fabrication of multifunctional flame retardant epoxy resin by a core–shell structural AgNC@boronate polymer. Chem. Eng. J. 2022, 438, 135402. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K. Structural transformation of layered double hydroxides: An in situ TEM analysis. NPJ 2D Mater. Appl. 2018, 2, 4. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Puglia, D.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Xia, L.; Zheng, X. Effect of a novel P/N/S-containing reactive flame retardant on curing behavior, thermal and flame-retardant properties of epoxy resin. J. Anal. Appl. Pyrolysis 2017, 127, 360–368. [Google Scholar] [CrossRef]
- Qiu, S.; Xing, W.; Feng, X.; Yu, B.; Mu, X.; Yuen, R.K.; Hu, Y. Self-standing cuprous oxide nanoparticles on silica@ polyphosphazene nanospheres: 3D nanostructure for enhancing the flame retardancy and toxic effluents elimination of epoxy resins via synergistic catalytic effect. Chem. Eng. J. 2017, 309, 802–814. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, K.K.R.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, e1805175. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, S.; Liu, S.; Zhao, J. Phosphorus-based triazine compound endowing epoxy thermosets with excellent flame retardancy and enhanced mechanical stiffness. Polym. Degrad. Stab. 2020, 180, 109293. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Q.-J.; Zeng, H.-Y.; Du, J.-Z.; Xu, S. Organic modification of Mo-decorated MgAl layered double hydroxide for polymer flame retardancy. Compos. Part A Appl. Sci. Manuf. 2019, 129, 105717. [Google Scholar] [CrossRef]
- Saritha, D.; Markandeya, Y.; Salagram, M.; Vithal, M.; Singh, A.K. Effect of Bi2O3 on physical, optical and structural studies of ZnO–Bi2O3–B2O3 glasses. J. Non-Cryst. Solids 2008, 354, 5573–5579. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Liao, M.-C.; Zeng, H.-Y.; Xu, S.; Liu, X.-J.; Du, J.-Z.; Zhu, P.-H.; Huang, Q.-J. Temperature effect on chromium(VI) removal by Mg/Al mixed metal oxides as adsorbents. Appl. Clay Sci. 2014, 102, 246–253. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, J.; Li, M.; Cai, Q.; Li, W.; Huang, C.; Yuan, C.; Xu, Y.; Zeng, B.; Dai, L. Design of h-BN@boronate polymer core-shell nanoplates to simultaneously enhance the flame retardancy and mechanical properties of epoxy resin through the interficial regulation. Compos. Part A Appl. Sci. Manuf. 2019, 130, 105751. [Google Scholar] [CrossRef]
- Chu, F.; Ma, C.; Zhang, T.; Xu, Z.; Mu, X.; Cai, W.; Zhou, X.; Ma, S.; Zhou, Y.; Hu, W.; et al. Renewable vanillin-based flame retardant toughening agent with ultra-low phosphorus loading for the fabrication of high-performance epoxy thermoset. Compos. Part B Eng. 2020, 190, 107925. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.H.; Song, C.F.; Chang, Y.; Chen, G.R.; Xu, Y.T.; Dai, L.Z. Modification of epoxy resin through the self-assembly of a surfactant-like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]















| DMA | The 3-Point Bending Test | ||||
|---|---|---|---|---|---|
| Samples | Tg (°C) | E’ (MPa) | tan δ | Flexural Strength (MPa) | Flexural Modulus (MPa) |
| EP | 159.0 | 1414.4 | 0.675 | 88.7 ± 4.0 | 2213.3 ± 44.1 |
| EP/LDH5 | 160.8 | 1350.8 | 0.604 | 90.2 ± 0.9 | 2528.5 ± 67.6 |
| EP/[email protected] | 167.7 | 1639.9 | 0.645 | 102.9 ± 3.4 | 2687.2 ± 100.4 |
| EP/LDH/BP5 | 174.5 | 1676.5 | 0.586 | 88.0 ± 1.8 | 2990.9 ± 255.7 |
| EP/LDH@BP5 | 175.2 | 1774.9 | 0.607 | 105.6 ± 1.2 | 3049.8 ± 167.9 |
| Samples | T5%/°C | T50%/°C | Tmax/°C | Char Yield /wt% (800 °C) |
|---|---|---|---|---|
| EP | 350.3 | 395.2 | 382.7 | 16.2 |
| EP/LDH5 | 335.1 | 387.8 | 377.5 | 18.6 |
| EP/[email protected] | 352.5 | 397.2 | 384.1 | 17.0 |
| EP/LDH/BP5 | 330.7 | 391.1 | 382.9 | 17.2 |
| EP/LDH@BP5 | 347.4 | 398.5 | 382.2 | 18.9 |
| Sample | PHRR | THR | SPR | TSP | COP |
|---|---|---|---|---|---|
| (kW/m2) | (MJ/m2) | (m2/s) | (m2) | (g/s) | |
| EP | 2010 | 113.7 | 0.370 | 23.41 | 0.0445 |
| EP/LDH5 | 1490 | 102.7 | 0.283 | 18.33 | 0.0262 |
| EP/LDH/BP5 | 1575 | 104.7 | 0.321 | 19.40 | 0.0379 |
| EP/LDH@BP5 | 1288 | 102.9 | 0.282 | 20.17 | 0.0290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.; He, S.; Peng, C.; Zeng, B.; Xia, L.; Miao, Z.; Xu, H.; Wang, S.; Chen, G.; Dai, L. LDH@Boronate Polymer Core–Shell Nanoparticles: Nanostructure Design for Synergistically Enhancing the Flame Retardancy of Epoxy Resin. Polymers 2023, 15, 2198. https://doi.org/10.3390/polym15092198
Chi C, He S, Peng C, Zeng B, Xia L, Miao Z, Xu H, Wang S, Chen G, Dai L. LDH@Boronate Polymer Core–Shell Nanoparticles: Nanostructure Design for Synergistically Enhancing the Flame Retardancy of Epoxy Resin. Polymers. 2023; 15(9):2198. https://doi.org/10.3390/polym15092198
Chicago/Turabian StyleChi, Cheng, Siyuan He, Chaohua Peng, Birong Zeng, Long Xia, Zhongxi Miao, Hui Xu, Shuchuan Wang, Guorong Chen, and Lizong Dai. 2023. "LDH@Boronate Polymer Core–Shell Nanoparticles: Nanostructure Design for Synergistically Enhancing the Flame Retardancy of Epoxy Resin" Polymers 15, no. 9: 2198. https://doi.org/10.3390/polym15092198
APA StyleChi, C., He, S., Peng, C., Zeng, B., Xia, L., Miao, Z., Xu, H., Wang, S., Chen, G., & Dai, L. (2023). LDH@Boronate Polymer Core–Shell Nanoparticles: Nanostructure Design for Synergistically Enhancing the Flame Retardancy of Epoxy Resin. Polymers, 15(9), 2198. https://doi.org/10.3390/polym15092198