Environment-Friendly Preparation and Characterization of Multilayered Conductive PVP/Col/CS Composite Doped with Nanoparticles as Potential Nerve Guide Conduits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Conductive Nanoparticles
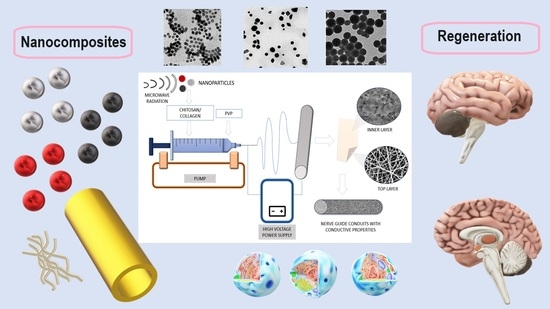
2.2.2. Preparation of NPs/PVP/ Col/CS Nanocomposites
2.2.3. Chemical Structure Study
2.2.4. Conductivity Study
2.2.5. TEM Analysis
2.2.6. SEM Analysis
2.2.7. Cytotoxicity Study
2.2.8. Biodegradation
- BD—biodegradation degree,
- W0—the initial weight,
- Wt—the weigh after time t.
2.2.9. Antibacterial Properties
3. Results
3.1. Nanoparticles Characteristics
3.2. FT-IR Chemical Structure Analysis
3.3. Conductivity Study

| Sample | Polymers | Nanoparticles |
|---|---|---|
| 1. | PVP | - |
| 2. | PVP/CS | - |
| 3. | PVP/Col/CS | - |
| 4. | PVP/Col/CS | Au |
| 5. | PVP/Col/CS | Ag |
| 6. | PVP/Col/CS | Pt |

3.4. SEM
3.5. Biodegradation




3.6. Determination of the Cytotoxicity of the Obtained Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, T. Bio-inspired artificial synapses: Neuromorphic computing chip engineering with soft biomaterials. Mem. Mater. Devices Circuits Syst. 2023, 6, 100088. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Aslam, M.A.; Bin Abdullah, M.F.; Hasan, A.; Shah, S.A.; Stojanović, G.M. Recent perspective of polymeric biomaterial in tissue engineering—A review. Mater. Today Chem. 2023, 34, 101818. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, H.; Wang, M.; Mantovani, D.; Yang, K.; Witte, F.; Tan, L.; Yue, B.; Qu, X. Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives. Innovation 2023, 4, 100503. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wise, S.G.; Kovacic, J.C.; Rnjak-Kovacina, J.; Lord, M.S. Biomaterials containing extracellular matrix molecules as biomimetic next-generation vascular grafts. Trends Biotechnol. 2023, 42, 369–381. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, Y. Advances in cell membrane-encapsulated biomaterials for tissue repair and regeneration. Appl. Mater. Today 2022, 26, 101389. [Google Scholar] [CrossRef]
- Elakkiya, S.; Arthanareeswaran, G.; Ismail, A.F.; Goh, P.S.; Lukka Thuyavan, Y. Review on characteristics of biomaterial and nanomaterials based polymeric nanocomposite membranes for seawater treatment application. Environ. Res. 2021, 197, 111177. [Google Scholar] [CrossRef]
- Lopes, L.M.; Germiniani, L.G.L.; Neto, J.B.M.R.; Andrade, P.F.; da Silveira, G.A.T.; Taketa, T.B.; Gonçalves, M.D.C.; Beppu, M.M. Preparation and characterization of porous membranes of glucomannan and silver decorated cellulose nanocrystals for application as biomaterial. Int. J. Biol. Macromol. 2023, 250, 126236. [Google Scholar] [CrossRef]
- Osorio, R.; Carrasco-Carmona, Á.; Toledano, M.; Osorio, E.; Medina-Castillo, A.L.; Iskandar, L.; Marques, A.; Deb, S.; Toledano-Osorio, M. Ex vivo investigations on bioinspired electrospun membranes as potential biomaterials for bone regeneration. J. Dent. 2020, 98, 103359. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, H.; Li, J.; Gu, R.; Dong, Y.; Zhao, Q.; Chen, Y.; Li, Y.; Wang, R. Antibacterial AgNPs-PAAm-CS-PVP nanocomposite hydrogel coating for urinary catheters. Eur. Polym. J. 2023, 196, 112260. [Google Scholar] [CrossRef]
- Bandatang, N.; Pongsomboon, S.-A.; Jumpapaeng, P.; Suwanakood, P.; Saengsuwan, S. Antimicrobial electrospun nanofiber mats of NaOH-hydrolyzed chitosan (HCS)/PVP/PVA incorporated with in-situ synthesized AgNPs: Fabrication, characterization, and antibacterial activity. Int. J. Biol. Macromol. 2021, 190, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Yimeng, W.; Yuan, Z.; Xuemin, L.; Qiqing, Z. The progress of biomaterials in peripheral nerve repair and regeneration. J. Neurorestoratol. 2020, 8, 252–269. [Google Scholar]
- Yukseloglu, S.M.; Sokmen, N.; Canoglu, S. Biomaterial applications of silk fibroin electrospun nanofibers. Microelectron. Eng. 2015, 146, 43–47. [Google Scholar] [CrossRef]
- Rahman, N.A.; Feisst, V.; Dickinson, M.E.; Malmström, J.; Dunbar, P.R.; Travas-Sejdic, J. Functional polyaniline nanofibre mats for human adipose-derived stem cell proliferation and adhesion. Mater. Chem. Phys. 2013, 138, 333–341. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Yuanhao, W.; Marriam, I.; Hu, J.; Tebyetekerwa, M.; Ramakrishna, S. Electrospun biomedical nanofibers and their future as intelligent biomaterials. Curr. Opin. Biomed. Eng. 2022, 24, 100418. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Zheng, X.; Yu, G.; Dan, N.; Dan, W.; Li, Z.; Chen, Y.; Liu, X. Origin of critical nature and stability enhancement in collagen matrix based biomaterials: Comprehensive modification technologies. Int. J. Biol. Macromol. 2022, 216, 741–756. [Google Scholar] [CrossRef]
- Huang, W.-H.; Ding, S.-L.; Zhao, X.-Y.; Li, K.; Guo, H.-T.; Zhang, M.-Z.; Gu, Q. Collagen for neural tissue engineering: Materials, strategies, and challenges. Mater. Today Bio 2023, 20, 100639. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2016, 3, 694–711. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mirhaj, M.; Labbaf, S.; Varshosaz, J.; Taymori, S.; Jafarpour, F.; Salehi, S.; Abadi, S.A.M.; Sepyani, A. Fabrication and evaluation of Cs/PVP sponge containing platelet-rich fibrin as a wound healing accelerator: An in vitro and in vivo study. Int. J. Biol. Macromol. 2022, 204, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Cao, H.; Tao, H.; Jin, L.; Luo, Y.; Tao, F.; Jiang, T. Applications of chitosan-based biomaterials: From preparation to spinal cord injury neuroprosthetic treatment. Int. J. Biol. Macromol. 2023, 230, 123447. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Z.; Zhang, H.; Yao, C. A review on chitosan-based biomaterial as carrier in tissue engineering and medical applications. Eur. Polym. J. 2023, 191, 112059. [Google Scholar] [CrossRef]
- Kantak, M.N.; Bharate, S.S. Analysis of clinical trials on biomaterial and therapeutic applications of chitosan: A review. Carbohydr. Polym. 2022, 278, 118999. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Nam, J.-P.; Nah, J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. Electrospraying: A facile technology unfolding the chitosan based drug delivery and biomedical applications. Eur. Polym. J. 2021, 147, 110326. [Google Scholar] [CrossRef]
- Hwang, M.; Karenson, M.O.; Elabd, Y.A. High Production Rate of High Purity, High Fidelity Nafion Nanofibers via Needleless Electrospinning. ACS Appl. Polym. Mater. 2019, 1, 2731–2740. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Uko, L.; Noby, H.; Zkria, A.; ElKady, M. Electrospraying of Bio-Based Chitosan Microcapsules Using Novel Mixed Cross-Linker: Experimental and Response Surface Methodology Optimization. Materials 2022, 15, 8447. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Isa, I.L.M.; Zaman, W.S.W.K.; Tabata, Y.; Fauzi, M.B. The Effect of Nanoparticle-Incorporated Natural-Based Biomaterials towards Cells on Activated Pathways: A Systematic Review. Polymers 2022, 14, 476. [Google Scholar] [CrossRef]
- Kumarage, V.; Siriwardane, I.W.; Sandaruwan, C.; Kandanapitiya, M.S.; Kottegoda, N.; Jayewardenepura, G. Nanotechnology Applications in Biomaterials; A review. J. Res. Technol. Eng. 2022, 3, 32–54. [Google Scholar]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Dhumale, V.A.; Gangwar, R.K.; Datar, S.S.; Sharma, R.B. Reversible Aggregation Control of Polyvinylpyrrolidone Capped Gold Nanoparticles as a Function of pH. Mater. Express 2012, 2, 311–318. [Google Scholar] [CrossRef]
- Boukhlifi, F. Quantitative Analysis by IR: Determination of Chitin/Chitosan DD. In Modern Spectroscopic Techniques and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- EN ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Polski Komitet Normalizacyjny: Warszawa, Polska, 2009.
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petiboi, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Tseomashko, N.; Tretyakova, A.; Abidova, A.; Butenko, I.; Pereyaslavtsev, A.; Arkharova, N.; Volkov, V.; Shtykova, E. Wound Coating Collagen-Based Composites with Ag Nanoparticles: Synthesis, Structure and Biological Activity. Coatings 2023, 13, 1315. [Google Scholar] [CrossRef]
- Pinho, A.C.; Fonseca, A.C.; Serra, A.C.; Santos, J.D.; Coelho, J.F.J. Peripheral Nerve Regeneration: Current Status and New Strategies Using Polymeric Materials. Adv. Health Mater. 2016, 5, 2732–2744. [Google Scholar] [CrossRef]
- Dai, J.; Gözaydın, G.; Hu, C.; Yan, N. Catalytic Conversion of Chitosan to Glucosaminic Acid by Tandem Hydrolysis and Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 12399–12407. [Google Scholar] [CrossRef]
- Roberts, S.E.; Thibaudeau, S.; Burrell, J.C.; Zager, E.L.; Cullen, D.K.; Levin, L.S. To reverse or not to reverse? A systematic review of autograft polarity on functional outcomes following peripheral nerve repair surgery. Microsurgery 2017, 37, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tagandurdyyeva, N.A.; Trube, M.A.; Shemyakin, I.O.; Solomitskiy, D.N.; Medvedev, G.V.; Dresvyanina, E.N.; Nashchekina, Y.A.; Ivan’kova, E.M.; Dobrovol’skaya, I.P.; Kamalov, A.M.; et al. Properties of Resorbable Conduits Based on Poly(L-Lactide) Nanofibers and Chitosan Fibers for Peripheral Nerve Regeneration. Polymers 2023, 15, 3323. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Mahady Dip, T.; Padhye, R.; Houshyar, S. Review on electrically conductive smart nerve guide conduit for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2023, 111, 1916–1950. [Google Scholar] [CrossRef] [PubMed]














| Parameter | Range |
|---|---|
| Temperature | 60–90 °C |
| Reaction time | 20–30 min |
| MW power | 10–25% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierakowska-Byczek, A.; Radwan-Pragłowska, J.; Janus, Ł.; Galek, T.; Łysiak, K.; Tupaj, M.; Bogdał, D. Environment-Friendly Preparation and Characterization of Multilayered Conductive PVP/Col/CS Composite Doped with Nanoparticles as Potential Nerve Guide Conduits. Polymers 2024, 16, 875. https://doi.org/10.3390/polym16070875
Sierakowska-Byczek A, Radwan-Pragłowska J, Janus Ł, Galek T, Łysiak K, Tupaj M, Bogdał D. Environment-Friendly Preparation and Characterization of Multilayered Conductive PVP/Col/CS Composite Doped with Nanoparticles as Potential Nerve Guide Conduits. Polymers. 2024; 16(7):875. https://doi.org/10.3390/polym16070875
Chicago/Turabian StyleSierakowska-Byczek, Aleksandra, Julia Radwan-Pragłowska, Łukasz Janus, Tomasz Galek, Karol Łysiak, Mirosław Tupaj, and Dariusz Bogdał. 2024. "Environment-Friendly Preparation and Characterization of Multilayered Conductive PVP/Col/CS Composite Doped with Nanoparticles as Potential Nerve Guide Conduits" Polymers 16, no. 7: 875. https://doi.org/10.3390/polym16070875
APA StyleSierakowska-Byczek, A., Radwan-Pragłowska, J., Janus, Ł., Galek, T., Łysiak, K., Tupaj, M., & Bogdał, D. (2024). Environment-Friendly Preparation and Characterization of Multilayered Conductive PVP/Col/CS Composite Doped with Nanoparticles as Potential Nerve Guide Conduits. Polymers, 16(7), 875. https://doi.org/10.3390/polym16070875