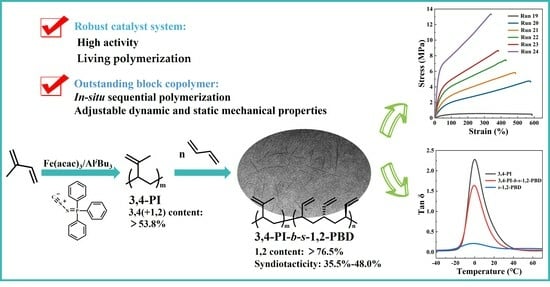
Preparation and Characterization of Soft-Hard Block Copolymer of 3,4-IP-b-s-1,2-PBD Using a Robust Iron-Based Catalyst System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization Procedure
2.3. Characterization
3. Results and Discussion
3.1. Homopolymerization Behavior of IP
3.2. Quasi-Living Polymerization of IP
3.3. Soft–Hard Block Copolymerization of 3,4-PI and s-1,2-PBD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hamley, I.W. Developments in Block Copolymer Science and Technology; Wiley Online Library: Hoboken, NJ, USA, 2004. [Google Scholar]
- Politakos, N.; Avgeropoulos, A. Advances and Applications of Block Copolymers. Polymers 2023, 15, 2930. [Google Scholar] [CrossRef] [PubMed]
- Beyer, V.P.; Kim, J.; Becer, C.R. Synthetic approaches for multiblock copolymers. Polym. Chem. 2020, 11, 1271–1291. [Google Scholar] [CrossRef]
- Schmalz, H.; Abetz, V. Block Copolymers with Crystallizable Blocks: Synthesis, Self-Assembly and Applications. Polymers 2022, 14, 696. [Google Scholar] [CrossRef] [PubMed]
- Kwag, G.; Kim, P.; Han, S.; Choi, H. Ultra high cis polybutadiene by monomeric neodymium catalyst and its tensile and dynamic properties. Polymer 2005, 46, 3782–3788. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, D.; Yan, X.; Gao, F.; Zhang, L.; Zhang, S.; Li, X. Cis-1,4-Polymerization of Isoprene by 1,3-Bis(oxazolinymethylidene)isoindoline-Ligated Rare-Earth Metal Dialkyl Complexes. Polymers 2017, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Kawahara, S.; Nishioka, H.; Yamano, M.; Yamamoto, Y. Synthetic rubber with the tensile strength of natural rubber. ACS Appl. Polym. Mater. 2022, 4, 2323–2328. [Google Scholar] [CrossRef]
- Peng, W.; Xie, J.; Zhang, J.; Yang, X.; He, A. Isoprene polymerizations catalyzed by TiCl4/MgCl2 type Ziegler-Natta catalysts with different titanium contents. Mol. Catal. 2020, 494, 111110. [Google Scholar] [CrossRef]
- Oburn, S.M.; Quentin, J.; Macgillivray, L.R. A Divergent Alkyne Diol Directs [2+2] Photoreactivity in the Solid State: Cocrystal, Supramolecular Catalysis, and Sublimation Effects. Molecules 2019, 24, 3059. [Google Scholar] [CrossRef]
- Ricci, G.; Pampaloni, G.; Sommazzi, A.; Masi, F. Dienes Polymerization: Where We Are and What Lies Ahead. Macromolecules 2021, 54, 5879–5914. [Google Scholar] [CrossRef]
- Halasa, A.F.; Hsu, W.-L.; Zanzig, D.J.; Allen, G.L.; Austin, L.E. Tire Tread Containing 3,4-Polyisoprene Rubber. CA2198563A1, 6 May 1997. [Google Scholar]
- Guo, J.; Zhang, S.; Ren, J.; Li, H.; Wang, S.; Hu, Y.; Zhou, G. Highly active and thermally robust pyridylbenzotriazole iron-based catalysts for preparation of polyisoprenes that feature high wet traction and low rolling resistance. Mol. Catal. 2023, 549, 113481. [Google Scholar] [CrossRef]
- Nakayama, Y.; Baba, Y.; Yasuda, H.; Kawakita, K.; Ueyama, N. Stereospecific Polymerizations of Conjugated Dienes by Single Site Iron Complexes Having Chelating N,N,N-Donor Ligands. Macromolecules 2003, 36, 7953–7958. [Google Scholar] [CrossRef]
- Wolpers, J.; Fuchs, H.B.; Herrmann, C.; Hellermann, W.; Nordsiek, K.-H. 3,4-Polyisoprene-Containing Rubber Blend Mixtures for Tire Treads. U.S. Patent 5,104,941, 14 April 1992. [Google Scholar]
- Ashitaka, H.; Ishikawa, H.; Ueno, H.; Nagasaka, A. Syndiotactic 1,2-polybutadiene with Co-CS2 catalyst system. I. Preparation, properties, and application of highly crystalline syndiotactic 1,2-polybutadiene. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 1853–1860. [Google Scholar] [CrossRef]
- Ichikawa, M.; Takeuchi, Y.; Kogure, A.; Kurita, H. Process for the Catalytic Preparation of 1,2-Polybutadiene Having a High Percentage of Vinyl Configuration. U.S. Patent 3,498,963, 3 March 1970. [Google Scholar]
- Monteil, V.; Bastero, A.; Mecking, S. 1,2-Polybutadiene Latices by Catalytic Polymerization in Aqueous Emulsion. Macromolecules 2005, 38, 5393–5399. [Google Scholar] [CrossRef]
- Tanaka, R.; Yuuya, K.; Sato, H.; Eberhardt, P.; Nakayama, Y.; Shiono, T. Synthesis of stereodiblock polyisoprene consisting of cis-1,4 and trans-1,4 sequences by using a neodymium catalyst: Change of the stereospecificity triggered by an aluminum compound. Polym. Chem. 2016, 7, 1239–1243. [Google Scholar] [CrossRef]
- Grune, E.; Johann, T.; Appold, M.; Wahlen, C.; Blankenburg, J.; Leibig, D.; Müller, A.H.E.; Gallei, M.; Frey, H. One-Step Block Copolymer Synthesis versus Sequential Monomer Addition: A Fundamental Study Reveals That One Methyl Group Makes a Difference. Macromolecules 2018, 51, 3527–3537. [Google Scholar] [CrossRef]
- Fu, T.; Jiang, L.; Sun, H.; Hou, Z.; Guo, F. Scandium-catalyzed stereoselective block and alternating copolymerization of diphenylphosphinostyrenes and isoprene. Polym. Chem. 2022, 13, 3498–3505. [Google Scholar] [CrossRef]
- Yao, C.; Liu, D.; Li, P.; Wu, C.; Li, S.; Liu, B.; Cui, D. Highly 3,4-Selective Living Polymerization of Isoprene and Copolymerization with ε-Caprolactone by an Amidino N-Heterocyclic Carbene Ligated Lutetium Bis (alkyl) Complex. Organometallics 2014, 33, 684–691. [Google Scholar] [CrossRef]
- Gong, D.; Weilun, Y.; Zhao, J.; Li, W.; Xu, Y.; Luo, Y.; Zhang, X.; Capacchione, C.; Grassi, A. Controlling external diphenylcyclohexylphosphine feeding to achieve cis-1,4-syn-1,2 sequence controlled polybutadienes via cobalt catalyzed 1,3-butadiene polymerization. J. Catal. 2019, 377, 367–377. [Google Scholar] [CrossRef]
- Cai, Z.; Shinzawa, M.; Nakayama, Y.; Shiono, T. Synthesis of Regioblock Polybutadiene with CoCl2-Based Catalyst via Reversible Coordination of Lewis Base. Macromolecules 2009, 42, 7642–7643. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, F.; Bi, J.; Zhang, H.; Zhang, C.; Hu, Y.; Bai, C.; Zhang, X. Synthesis and Characterization of Soft-Hard Stereoblock Polybutadiene with Fe(2-EHA)3/Al(i-Bu)3/DEP Catalyst System. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1182–1188. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Bertini, F.; Porri, L. 1,2 Syndiotactic polybutadiene of controlled crystallinity by butadiene-isoprene copolymerization with CrCl2·(dmpe)2-MAO. Polym. Bull. 2002, 48, 25–31. [Google Scholar] [CrossRef]
- Nath, D.C.D.; Shiono, T.; Ikeda, T. Copolymerization of 1,3-Butadiene and Isoprene with Cobalt Dichloride/Methylaluminoxane in the Presence of Triphenylphosphine. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3086–3092. [Google Scholar] [CrossRef]
- Li, P.; Liu, K.; Fu, Z.; Yu, Y.; Wang, Z.; Hua, J. Preparation of Butadiene-Isoprene Copolymer with High Vinyl Contents by Al(OPhCH3)(i-Bu)2/MoO2Cl2∙TNPP. Polymers 2019, 11, 527. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, H.; Wang, X.; Jiang, Z.; Gu, J.; Liu, H.; Zhang, C.; Yang, Q.; Zhang, X. Novel butadiene/isoprene copolymer with predominant 1,2/3,4-units: A tough thermoplastic elastomer material with superior dynamic mechanical properties. Eur. Polym. J. 2023, 196, 112333. [Google Scholar] [CrossRef]
- Porri, L.; Giarrusso, A. Conjugated Diene Polymerization. Compr. Polym. Sci. Suppl. 1989, 4, 53–108. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Sun, G.; Liu, J.; Wang, M.; Li, S.; Cui, D. 3,4-Polymerization of Isoprene by Using NSN- and NPN-Ligated Rare Earth Metal Precursors: Switching of Stereo Selectivity and Mechanism. Macromolecules 2014, 47, 4971–4978. [Google Scholar] [CrossRef]
- Forens, A.; Roos, K.; Gadenne, B.; Carlotti, S. Anionic polymerization of butadiene by dialkylmagnesium / alkali metal alkoxide systems in apolar medium: Polybutadiene microstructure and polymerization control. Polymer 2020, 205, 122864. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, Y.; Hu, W.; Luo, W.; Zhao, Y.; Huang, W.; Gong, D. Synthesis and properties of syndiotactic 1,2-polybutadiene catalyzed by iron catalyst with phosphate as additive. J. Appl. Polym. Sci. 2020, 138, 49686. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Zanchin, G.; Palucci, B.; Boccia, A.C.; Sommazzi, A.; Masi, F.; Zacchini, S.; Guelfi, M.; Pampaloni, G. Highly Stereoregular 1,3-Butadiene and Isoprene Polymers through Monoalkyl-N-Aryl-Substituted Iminopyridine Iron Complex-Based Catalysts: Synthesis and Characterization. Macromolecules 2021, 54, 9947–9959. [Google Scholar] [CrossRef]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-Catalyzed Polymerization of Isoprene and Other 1,3-Dienes. Angew. Chem. 2012, 124, 11975–11978. [Google Scholar] [CrossRef]
- Zhang, L.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z. Isoprene Polymerization with Yttrium Amidinate Catalysts: Switching the Regio- and Stereoselectivity by Addition of AlMe3. Angew. Chem. 2008, 120, 2682–2685. [Google Scholar] [CrossRef]
- Li, S.; Cui, D.; Li, D.; Hou, Z. Highly 3,4-Selective Polymerization of Isoprene with NPN Ligand Stabilized Rare-Earth Metal Bis(alkyl)s. Structures and Performances. Organometallics 2009, 28, 4814–4822. [Google Scholar] [CrossRef]
- Yu, X.; Li, M.; Hong, J.; Zhou, X.; Zhang, L. Living 3,4-(Co)Polymerization of Isoprene/Myrcene and One-Pot Synthesis of a Polyisoprene Blend Catalyzed by Binuclear Rare-Earth Metal Amidinate Complexes. Chem.—A Eur. J. 2019, 25, 2569–2576. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, S.; Zhu, X.; Zhou, S.; Wei, Y.; Huang, Z.; Mu, X. Synthesis and Characterization of Rare-Earth Metal Complexes Supported by 2-Imino or Amino Appended Indolyl Ligands with Diverse Hapticities: Tunable Selective Catalysis for Isoprene Polymerization. Organometallics 2017, 36, 3812–3822. [Google Scholar] [CrossRef]
- Ricci, G.; Battistella, M.; Porri, L. Chemoselectivity and Stereospecificity of Chromium (II) Catalysts for 1,3-Diene Polymerization. Macromolecules 2001, 34, 5766–5769. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Wang, L.; Zhu, G.; Kuang, J.; Zhu, G.; Xu, G.; Wang, Q. 3,4-Enhanced Polymerization of Isoprene Catalyzed by Side-Arm Tridentate Iminopyridine Iron Complex with High Activity: Optimization via Response Surface Methodology. Polymers 2023, 15, 1231. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, Y.; Yu, Y.; Yang, Q.; Na, L.; Liu, H.; Zhang, C.; Zhang, X. Study on Catalytic Behavior of Iron-based Catalyst with IITP as Electron Donor in the Polymerization of Butadiene. Chin. J. Polym. Sci. 2024. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Hu, Y.; Zhang, X. Effects of Crystal Growth Conditions on Morphology of Crystalline Syndiotactic 1,2-Polybutadiene. Cryst. Growth Des. 2003, 4, 117–121. [Google Scholar] [CrossRef]
- Huang, C.I.; Chang, C.P.; Shimizu, K.; Han, C.C. Phase behavior and crystallization analysis in binary crystalline blends of syndiotactic polypropylene and ethylene-Propylene random copolymer. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2995–3005. [Google Scholar] [CrossRef]
- ISO 37:2011; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2011.
- Fischbach, A.; Anwander, R. Rare-Earth Metals and Aluminum Getting Close in Ziegler-Type Organometallics. In Neodymium Based Ziegler Catalysts–Fundamental Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; Volume 204, pp. 155–281. [Google Scholar]
- Swift, H.E.; Bozik, J.E.; Wu, C.Y. Specific Catalysis with Iron Coordination Complexes. J. Catal. 1970, 17, 331–340. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. In Neodymium Based Ziegler Catalysts–Fundamental Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–154. [Google Scholar]
- Burcher, B.; Breuil, P.A.R.; Magna, L.; Olivier-Bourbigou, H. Iron-Catalyzed Oligomerization and Polymerization Reactions; Springer: Berlin/Heidelberg, Germany, 2015; Volume 50, pp. 217–257. [Google Scholar]
- Zhang, Z.Y.; Zhang, H.J.; Ma, H.M.; Wu, Y. A novel iron catalyst for the polymerization of butadiene. J. Mol. Catal. 1982, 17, 65–76. [Google Scholar] [CrossRef]
- Gong, D.; Dong, W.; Hu, J.; Zhang, X.; Jiang, L. Living polymerization of 1,3-butadiene by a Ziegler–Natta type catalyst composed of iron(III) 2-ethylhexanoate, triisobutylaluminum and diethyl phosphite. Polymer 2009, 50, 2826–2829. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Bar, G.; Dosière, M.; Koch, M.H.J. A Novel View on Crystallization and Melting of Semirigid Chain Polymers: The Case of Poly(trimethylene terephthalate). Macromolecules 2008, 41, 9224–9233. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P. The Structure of Crystalline 1,2-Polybutadiene and of Other “Syndyotactic Polymers”. J. Polym Sci. 1956, 20, 251–266. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Y.; Ying, W.; Hu, Z.; Luo, W.; Tang, F.; Huang, W.; Jia, X.; Gong, D. 1,2-Syndiotactic polymerization of butadiene catalyzed by iron (III) acetylacetonate in combination with exogenous phosphate. Mol. Catal. 2020, 497, 111219. [Google Scholar] [CrossRef]
- Rangarajan, P.; Register, R.A.; Fetters, L.J. Morphology of Semicrystalline Block Copolymers of Ethylene-(Ethylene-alt-propylene). Macromolecules 2002, 26, 4640–4645. [Google Scholar] [CrossRef]
- Koo, C.M.; Wu, L.; Lim, L.S.; Mahanthappa, M.K.; Hillmyer, M.A.; Bates, F.S. Microstructure and Mechanical Properties of Semicrystalline-Rubbery-Semicrystalline Triblock Copolymers. Macromolecules 2005, 38, 6090–6098. [Google Scholar] [CrossRef]
- Rangarajan, P.; Register, R.A.; Adamson, D.H.; Fetters, L.J.; Bras, W.; Naylor, S.; Ryan, A.J. Dynamics of Structure Formation in Crystallizable Block Copolymers. Macromolecules 2002, 28, 1422–1428. [Google Scholar] [CrossRef]
- Baklanova, O.N.; Knyazheva, O.A.; Lavrenov, A.V.; Drozdov, V.A.; Trenikhin, M.V. Effect of the aggregates size and oxygen content of carbon black on elastic characteristics of rubber. Polym. Bull. 2021, 79, 9503–9521. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, M.; Zhu, X.; Ren, Z.; Hu, Y.; Zhao, H.; Wang, W.; Chen, Y.; Gao, K.; Zhou, Y. Expanding the “Magic Triangle” of Reinforced Rubber Using a Supramolecular Filler Strategy. Materials 2023, 16, 3429. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tian, Q.; Liu, Y.; Wang, Y.; Li, X.; Zhang, Z.; Ding, T. Application of carboxylated ethylene/vinyl acetate copolymer-modified nanosilica in tire tread rubber. Iran. Polym. J. 2020, 29, 853–864. [Google Scholar] [CrossRef]







| Run | Cocat. | [Al]/[Fe] | [IP]/[Fe] | Yield (wt%) | Mnb (×104) | PDI b | Microstructure c (%) | Tgd (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 3,4 | 1,2 | 1,4 | ||||||||
| 1 | AliBu3 | 10 | 15,000 | 94.6 | 73.1 | 1.97 | 51.6 | 3.0 | 45.4 | −16.6 |
| 2 | AliBu3 | 20 | 15,000 | 97.7 | 91.3 | 1.92 | 51.2 | 3.2 | 45.6 | −15.8 |
| 3 | AliBu3 | 30 | 15,000 | 97.1 | 82.5 | 1.92 | 51.1 | 3.1 | 45.8 | −17.6 |
| 4 | AlEt3 | 10 | 15,000 | trace | -- | -- | -- | -- | -- | -- |
| 5 | AlEt3 | 20 | 15,000 | 95.8 | 65.0 | 2.03 | 51.1 | 2.6 | 46.3 | −17.2 |
| 6 | AlEt3 | 30 | 15,000 | 88.3 | 75.5 | 1.90 | 52.1 | 3.0 | 44.9 | −16.6 |
| 7 | AliBu2H | 10 | 15,000 | 15.1 | 45.0 | 2.01 | 51.8 | 5.2 | 43.0 | −14.1 |
| 8 | AliBu2H | 20 | 15,000 | 95.9 | 64.5 | 2.03 | 48.8 | 5.0 | 46.2 | −16.0 |
| 9 | AliBu2H | 30 | 15,000 | 90.4 | 69.0 | 2.20 | 49.4 | 5.1 | 45.5 | −16.7 |
| 10 | AliBu3 | 20 | 10,000 | 99.8 | 70.2 | 1.94 | 51.2 | 2.6 | 46.2 | −17.3 |
| 11 | AliBu3 | 20 | 30,000 | 72.0 | 100.3 | 1.88 | 50.3 | 3.7 | 46.0 | −16.3 |
| 12 | AliBu3 | 20 | 50,000 | 42.0 | 102.7 | 1.88 | 50.8 | 3.9 | 45.3 | −16.1 |
 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | [IP]/[BD] Feed Ratio | Yield (%) | Mn b (×104) | PDI b | PI Microstructure (%) c | PBD Microstructure (%) c | BD in Copolymer c (mol%) | rrrr d (%) | Tg e (°C) | Tm e (°C) | Xc f (%) | |||
| 3,4 | 1,2 | 1,4 | 1,2 | 1,4 | ||||||||||
| 19 | 500:0 | 100 | 19.9 | 2.02 | 52.1 | 2.1 | 45.8 | -- | -- | 0.0 | -- | −17.5 | -- | -- |
| 20 | 500:500 | 98.4 | 27.0 | 2.35 | 50.3 | 3.5 | 46.2 | 77.7 | 22.3 | 45.8 | 35.5 | −17.5 | 99.7 | 10.5 |
| 21 | 500:750 | 97.4 | 35.8 | 2.43 | 49.3 | 5.0 | 45.8 | 76.9 | 23.1 | 51.9 | 38.1 | −17.6 | 100.7 | 11.2 |
| 22 | 500:1000 | 97.2 | 39.7 | 2.51 | 46.9 | 6.1 | 47.0 | 76.5 | 23.5 | 60.7 | 44.4 | −17.9 | 100.7 | 16.9 |
| 23 | 500:1500 | 99.0 | 46.1 | 2.44 | 46.8 | 5.6 | 47.6 | 77.9 | 22.1 | 68.6 | 48.0 | −18.3 | 99.7 | 18.1 |
| 24 | 0:500 | 96.3 | 12.4 | 2.46 | -- | -- | -- | 64.9 | 35.1 | 100 | 68.0 | −22.3 | 90.2 | 24.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xu, S.; Yu, Y.; Liu, H.; Wang, F.; Na, L.; Yang, Q.; Zhang, C.; Zhang, X. Preparation and Characterization of Soft-Hard Block Copolymer of 3,4-IP-b-s-1,2-PBD Using a Robust Iron-Based Catalyst System. Polymers 2024, 16, 1172. https://doi.org/10.3390/polym16081172
Zhao Y, Xu S, Yu Y, Liu H, Wang F, Na L, Yang Q, Zhang C, Zhang X. Preparation and Characterization of Soft-Hard Block Copolymer of 3,4-IP-b-s-1,2-PBD Using a Robust Iron-Based Catalyst System. Polymers. 2024; 16(8):1172. https://doi.org/10.3390/polym16081172
Chicago/Turabian StyleZhao, Yingnan, Shiliang Xu, Yao Yu, Heng Liu, Feng Wang, Lihua Na, Qi Yang, Chunyu Zhang, and Xuequan Zhang. 2024. "Preparation and Characterization of Soft-Hard Block Copolymer of 3,4-IP-b-s-1,2-PBD Using a Robust Iron-Based Catalyst System" Polymers 16, no. 8: 1172. https://doi.org/10.3390/polym16081172
APA StyleZhao, Y., Xu, S., Yu, Y., Liu, H., Wang, F., Na, L., Yang, Q., Zhang, C., & Zhang, X. (2024). Preparation and Characterization of Soft-Hard Block Copolymer of 3,4-IP-b-s-1,2-PBD Using a Robust Iron-Based Catalyst System. Polymers, 16(8), 1172. https://doi.org/10.3390/polym16081172