Cationic Azobenzenes as Light-Responsive Crosslinkers for Alginate-Based Supramolecular Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of AZO A
2.1.1. Synthesis of 4,4′-(Diazene-1,2-diyl)diphenol (1 in Figure S1)
2.1.2. Synthesis of 1,2-Bis(4-((6-bromohexyl)oxy)phenyl)diazene (2 in Figure S1)
2.1.3. Synthesis of 6,6′-((Diazene-1,2-diylbis(4,1-phenylene))bis(oxy))bis(N,N,N-trimethylhexan-1-aminium) (AZO A, Figure S1)
2.2. Synthesis of AZO B
Synthesis of 1,1′-(((Diazene-1,2-diylbis(4,1-phenylene))bis(oxy))bis(hexane 6,1diyl))bis(pyridin-1-ium) (AZO B, in Figure S1)
2.3. Synthesis of AZO C
2.3.1. Synthesis of N-(4-((4-hydroxyphenyl)diazenyl)phenyl)acetamide (1′ in Figure S2)
2.3.2. Synthesis of N-(4-((4-(4-bromobutoxy)phenyl)diazenyl)phenyl)acetamide (2′ in Figure S2)
2.3.3. Synthesis of 4-(4-((4-Acetamidophenyl)diazenyl)phenoxy)-N,N,N-trimethylbutan-1-aminium (3′ in Figure S2)
2.3.4. Synthesis of 4-(4-((4-Aminophenyl)diazenyl)phenoxy)-N,N,N-trimethylbutan-1-aminium (AZO C)
2.4. Hydrogel Preparation
3. Results and Discussion
3.1. Synthesis of Ionic Azobenzenes
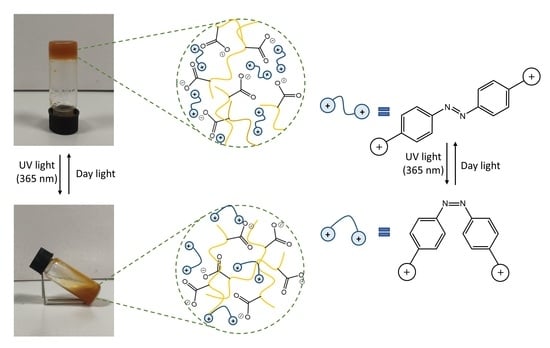
3.2. Light-Responsive Behavior of Azobenzenes
3.3. Hydrogel Formation and pH-Responsive Properties
3.4. Hydrogel Photoresponsive Properties
3.5. FT-IR Characterization of the Hydrogels
3.6. Hydrogel Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef]
- Karimi, S.; Rasuli, H.; Mohammadi, R. Facile preparation of pH-sensitive biocompatible alginate beads havening layered double hydroxide supported metal-organic framework for controlled release from doxorubicin to breast cancer cells. Int. J. Biol. Macromol. 2023, 234, 123538. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R. Smart biomaterials: Recent advances and future directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2024, 24, e202300217. [Google Scholar] [CrossRef]
- Di Martino, M.; Sessa, L.; Diana, R.; Piotto, S.; Concilio, S. Recent Progress in Photoresponsive Biomaterials. Molecules 2023, 28, 3712. [Google Scholar] [CrossRef]
- Dudek, M.; Pokładek, Z.; Deiana, M.; Matczyszyn, K. Molecular design and structural characterization of photoresponsive azobenzene-based polyamide units. Dye. Pigment. 2020, 180, 108501. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S.; Xu, W.-C.; Xu, G.; Wu, S. Photoresponsive polymers with multi-azobenzene groups. Polym. Chem. 2019, 10, 4389–4401. [Google Scholar] [CrossRef]
- Giménez, V.M.M.; Arya, G.; Zucchi, I.A.; Galante, M.J.; Manucha, W. Photo-responsive polymeric nanocarriers for target-specific and controlled drug delivery. Soft Matter 2021, 17, 8577–8584. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed]
- Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Advances and opportunities in the exciting world of azobenzenes. Nat. Rev. Chem. 2022, 6, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, D.; Shinde, V.V.; Hu, Y.; Kim, C.; Jung, S. Azobenzene-grafted carboxymethyl cellulose hydrogels with photo-switchable, reduction-responsive and self-healing properties for a controlled drug release system. Int. J. Biol. Macromol. 2020, 163, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Rodell, C.B.; Chen, M.H.; Morrow, M.G.; Anseth, K.S.; Burdick, J.A. Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjugate Chem. 2018, 29, 905–913. [Google Scholar] [CrossRef]
- Yang, R.; Peng, S.; Wan, W.; Hughes, T.C. Azobenzene based multistimuli responsive supramolecular hydrogels. J. Mater. Chem. C 2014, 2, 9122–9131. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Marturano, V.; Manganelli, M.; Santillo, C.; Ambrogi, V.; Filippone, G.; Cerruti, P. Light-responsive and self-healing behavior of azobenzene-based supramolecular hydrogels. J. Colloid Interface Sci. 2020, 568, 16–24. [Google Scholar] [CrossRef]
- Karcher, J.; Kirchner, S.; Leistner, A.-L.; Hald, C.; Geng, P.; Bantle, T.; Gödtel, P.; Pfeifer, J.; Pianowski, Z.L. Selective release of a potent anticancer agent from a supramolecular hydrogel using green light. RSC Adv. 2021, 11, 8546–8551. [Google Scholar] [CrossRef]
- Lv, J.-A.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef]
- Xu, G.; Li, S.; Liu, C.; Wu, S. Photoswitchable Adhesives Using Azobenzene-Containing Materials. Chem. Asian J. 2020, 15, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Sessa, L.; Di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Azobenzene as Antimicrobial Molecules. Molecules 2022, 27, 5643. [Google Scholar] [CrossRef]
- Peng, K.; Zheng, L.; Zhou, T.; Zhang, C.; Li, H. Light manipulation for fabrication of hydrogels and their biological applications. Acta Biomater. 2022, 137, 20–43. [Google Scholar] [CrossRef]
- Sessa, L.; Concilio, S.; Iannelli, P.; De Santis, F.; Porta, A.; Piotto, S. Antimicrobial azobenzene compounds and their potential use in biomaterials. In Proceedings of the AIP Conference Proceedings, Tokyo, Japan, 1–2 November 2016. [Google Scholar]
- Wang, T.; Gao, F.; Zhang, Y.; Wang, S.; Li, X.; Zhao, Z.; Dong, Y.; Li, X. Construction of multiresponsive supramacromolecular hydrogels with a novel azobenzene derivative as crosslinkers. J. Mater. Res. Technol. 2023, 22, 1781–1790. [Google Scholar] [CrossRef]
- Gao, F.; Bi, Z.; Wang, S.; Zhao, Z.; Dong, Y.; Li, X. An amphiphilic azobenzene derivative as a crosslinker in the construction of smart supramacromolecular hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129088. [Google Scholar] [CrossRef]
- Leistner, A.-L.; Kistner, D.G.; Fengler, C.; Pianowski, Z.L. Reversible photodissipation of composite photochromic azobenzene-alginate supramolecular hydrogels. RSC Adv. 2022, 12, 4771–4776. [Google Scholar] [CrossRef]
- Yang, R.; Jin, W.; Huang, C.; Liu, Y. Azobenzene Based Photo-Responsive Hydrogel: Synthesis, Self-Assembly, and Antimicrobial Activity. Gels 2022, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhang, Y.; Luan, T.; Wang, Z.; Tang, R.; Xing, P.; Hao, A. Hydrogels Self-Assembled from an Azobenzene Building Block: Stability toward UV Irradiation in the Gel and Thin-Film States. Chempluschem 2019, 84, 328–332. [Google Scholar] [CrossRef]
- Heeres, A.; van der Pol, C.; Stuart, M.; Friggeri, A.; Feringa, B.L.; van Esch, J. Orthogonal Self-Assembly of Low Molecular Weight Hydrogelators and Surfactants. J. Am. Chem. Soc. 2003, 125, 14252–14253. [Google Scholar] [CrossRef]
- Omar, J.; Ponsford, D.; Dreiss, C.A.; Lee, T.C.; Loh, X.J. Supramolecular hydrogels: Design strategies and contemporary biomedical applications. Chem. Asian J. 2022, 17, e202200081. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Sun, F.; Zhang, D.; Zhang, G.; Huang, Y.; Zhao, R.; Zhu, D. Multistimuli Responsive Organogels Based on a New Gelator Featuring Tetrathiafulvalene and Azobenzene Groups: Reversible Tuning of the Gel−Sol Transition by Redox Reactions and Light Irradiation. J. Am. Chem. Soc. 2010, 132, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Yeap, G.-Y.; Mahmood, W.A.K.; Tan, P.-L.; Cheong, K.-Y. Thermal and photo reversible gel–sol transition of azobenzene based liquid crystalline organogel. J. Photochem. Photobiol. A Chem. 2014, 278, 19–24. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.; Lee, J.; Malpani, Y.; Jung, Y.-S.; Kang, B.; Lee, J.Y.; Ozasa, K.; Isoshima, T.; Lee, S.Y.; et al. Stimulus-Responsive Azobenzene Supramolecules: Fibers, Gels, and Hollow Spheres. Langmuir 2013, 29, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, Z.; Xu, Y.; Shi, J.; Lin, H.; Zhang, Y. Supramolecular hydrogels based on short peptides linked with conformational switch. Org. Biomol. Chem. 2011, 9, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qiao, Y.; Tang, P.; Li, Z.; Huang, J. Controllable self-assembled laminated nanoribbons from dipeptide-amphiphile bearing azobenzene moiety. Soft Matter 2011, 7, 2762–2769. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Tamaoki, N. Photoisomerization of Azobenzene Units Controls the Reversible Dispersion and Reorganization of Fibrous Self-Assembled Systems. J. Phys. Chem. B 2010, 114, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Yoshiyama, C.; Kitaoka, T. Helical Assembly of Azobenzene-Conjugated Carbohydrate Hydrogelators with Specific Affinity for Lectins. Langmuir 2012, 28, 4404–4412. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zou, W.; Julita, V.; Ramanathan, R.; Tabor, R.F.; Nixon-Luke, R.; Bryant, G.; Bansal, V.; Wilkinson, B.L. Photomodulation of bacterial growth and biofilm formation using carbohydrate-based surfactants. Chem. Sci. 2016, 7, 6628–6634. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Guo, Q.; Hughes, T.C.; Hartley, P.G. Reversible Photorheological Lyotropic Liquid Crystals. Langmuir 2014, 30, 866–872. [Google Scholar] [CrossRef]
- Yin, C.; Jiang, F.; Li, B.; Wu, L. Multiple modulations for supramolecular hydrogels of bola-form surfactants bearing rigid and flexible groups. Soft Matter 2019, 15, 5034–5041. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chu, C.-C. Synthesis of photoresponsive hybrid alginate hydrogel with photo-controlled release behavior. Carbohydr. Polym. 2015, 119, 18–25. [Google Scholar] [CrossRef]
- Xie, M.; Wu, C.; Chen, C.; Liu, Y.; Zhao, C. Photo-adaptable shape memory hydrogels based on orthogonal supramolecular interactions. Polym. Chem. 2019, 10, 4852–4858. [Google Scholar] [CrossRef]
- He, F.; Wang, L.; Yang, S.; Qin, W.; Feng, Y.; Liu, Y.; Zhou, Y.; Yu, G.; Li, J. Highly stretchable and tough alginate-based cyclodextrin/Azo-polyacrylamide interpenetrating network hydrogel with self-healing properties. Carbohydr. Polym. 2021, 256, 117595. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, T.; Miyauchi, M.; Kawaguchi, Y.; Yamaguchi, H.; Harada, A. Complex formation and gelation between copolymers containing pendant azobenzene groups and cyclodextrin polymers. Chem. Lett. 2004, 33, 890–891. [Google Scholar] [CrossRef]
- Ma, X.; He, L.; Wan, X.; Xiang, S.; Fan, Y.; Xiong, X.; Gan, L.; Huang, J. Reversible mechanical regulation and splicing ability of alginate-based gel based on photo-responsiveness of molecular-level conformation. Materials 2019, 12, 2919. [Google Scholar] [CrossRef]
- Concilio, S.; Sessa, L.; Petrone, A.M.; Porta, A.; Diana, R.; Iannelli, P.; Piotto, S. Structure Modification of an Active Azo-Compound as a Route to New Antimicrobial Compounds. Molecules 2017, 22, 875. [Google Scholar] [CrossRef]
- Zinchenko, A.A.; Tanahashi, M.; Murata, S. Photochemical modulation of DNA conformation by organic dications. ChemBioChem 2012, 13, 105–111. [Google Scholar] [CrossRef]
- Mutter, N.L.; Volarić, J.; Szymanski, W.; Feringa, B.L.; Maglia, G. Reversible Photocontrolled Nanopore Assembly. J. Am. Chem. Soc. 2019, 141, 14356–14363. [Google Scholar] [CrossRef]
- Blevins, A.A.; Blanchard, G.J. Effect of Positional Substitution on the Optical Response of Symmetrically Disubstituted Azobenzene Derivatives. J. Phys. Chem. B 2004, 108, 4962–4968. [Google Scholar] [CrossRef]
- Maity, C.; Das, N. Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives. Top. Curr. Chem. 2021, 380, 3. [Google Scholar] [CrossRef]
- Afzal, S.; Maswal, M.; Dar, A.A. Rheological behavior of pH responsive composite hydrogels of chitosan and alginate: Characterization and its use in encapsulation of citral. Colloids Surf. B Biointerfaces 2018, 169, 99–106. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Omer, A.M.; Wassel, M.A.; Tamer, T.M.; Abd Elmonem, M.S.; Ibrahim, S.A. Novel smart pH sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: I. Preparation and characterization. Int. J. Pharm. Pharm. Sci. 2015, 7, 320–326. [Google Scholar]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Wan, T.; Xu, H.; Yuan, Y.; He, W. Preparation and photochemical behavior of a cationic azobenzene dye-montmorillonite intercalation compound. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2007, 22, 466–469. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Pourbadiei, B.; Adlsadabad, S.Y.; Rahbariasr, N.; Pourjavadi, A. Synthesis and characterization of dual light/temperature-responsive supramolecular injectable hydrogel based on host-guest interaction between azobenzene and starch-grafted β-cyclodextrin: Melanoma therapy with paclitaxel. Carbohydr. Polym. 2023, 313, 120667. [Google Scholar] [CrossRef]










| % wt SA_% wt AZO | 8%/0.5% | 8%/1% | 5%/0.5% | 5%/1% |
|---|---|---|---|---|
| SA_AZO A | V | V | L | L |
| SA_AZO B | V | V | L | L |
| SA_AZO C | P | P | P | P |
| % wt SA_% wt AZO | 8%/0.5% | 8%/1% | 5%/0.5% | 5%/1% | 2%/0.5% | 2%/1% |
|---|---|---|---|---|---|---|
| SA_AZO A | G (2) | G (4) | L (1) | G (3) | * | * |
| SA_AZO B | * | * | G (5) | G (6) | L (7) | V (8) |
| SA_AZO C | * | * | G (9) | G (10) | L (11) | L (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Martino, M.; Sessa, L.; Panunzi, B.; Diana, R.; Piotto, S.; Concilio, S. Cationic Azobenzenes as Light-Responsive Crosslinkers for Alginate-Based Supramolecular Hydrogels. Polymers 2024, 16, 1233. https://doi.org/10.3390/polym16091233
Di Martino M, Sessa L, Panunzi B, Diana R, Piotto S, Concilio S. Cationic Azobenzenes as Light-Responsive Crosslinkers for Alginate-Based Supramolecular Hydrogels. Polymers. 2024; 16(9):1233. https://doi.org/10.3390/polym16091233
Chicago/Turabian StyleDi Martino, Miriam, Lucia Sessa, Barbara Panunzi, Rosita Diana, Stefano Piotto, and Simona Concilio. 2024. "Cationic Azobenzenes as Light-Responsive Crosslinkers for Alginate-Based Supramolecular Hydrogels" Polymers 16, no. 9: 1233. https://doi.org/10.3390/polym16091233
APA StyleDi Martino, M., Sessa, L., Panunzi, B., Diana, R., Piotto, S., & Concilio, S. (2024). Cationic Azobenzenes as Light-Responsive Crosslinkers for Alginate-Based Supramolecular Hydrogels. Polymers, 16(9), 1233. https://doi.org/10.3390/polym16091233