Enhanced Photophysical Properties of Nanopatterned Titania Nanodots/Nanowires upon Hybridization with Silica via Block Copolymer Templated Sol-Gel Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scheme

2.2. Chemical Structure

2.3. Morphology Study


2.4. UV-visible Absorption

2.5. FTIR Spectrum

2.6. Photocatalysis

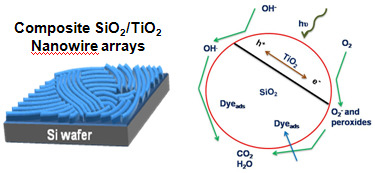
2.7. Mechanism of the Enhancement of Photocatalytic Activity

3. Experimental Section
3.1. Materials
3.2. TiO2 Sol-gel Precursor Solution
3.3. SiO2-TiO2 Sol-gel Precursor Synthesis
3.4. Film Preparation
3.5. Characterization
4. Conclusions
Acknowledgements
References
- Linsebigler, A.L.; Guangquan, L.; John, T.Y. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Brown, G.E.; Henrich, V.E.; Casey, W.H.; Clark, D.L.; Eggleston, C.; Femly, A.; Goodman, G.W.; Gretzel, M.; Macial, G.; McGarthy, M.I.; Nealson, K.H.; Sverjensky, D.A.; Toney, M.F.; Zachara, J.M. Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chem. Rev. 1999, 99, 77–174. [Google Scholar] [CrossRef] [PubMed]
- Bilmes, S.A.; Mandelbaum, P.; Alvarez, F.; Victoria, N.M. Surface and electronic structure of titanium dioxide photocatalysts. J. Phys. Chem. B 2000, 104, 9851–9858. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Huogen, Y.; Jiaguo, Y.; Bei, C.; Shengwei, L. Novel preparation and photocatalytic activity of one-dimensional TiO2 hollow structures. Nanotechnology 2007, 18, 065604. [Google Scholar] [CrossRef]
- Xiwang, Z.; Jia, H.P.; Alan, J.D.; Weijiong, F.; Darren, D.S.; James, O.L. Combination of one-dimensional TiO2 nanowire photocatalytic oxidation with microfiltration for water treatment. Water Res. 2009, 43, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Mingxia, X.; Qinglin, Z.; Hongxing, L.; Guozhang, D.; Hongchun, Y.; Taihong, W.; Bingsuo, Z.; Yanguo, W. The large-scale synthesis of one-dimensional TiO2 nanostructures using palladium as catalyst at low temperature. Nanotechnology 2009, 20, 055605. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Murayama, H.; Nagai, A.; Shimada, A.; Hatamachi, T.; Kodama, T.; Kitayama, Y. Degradation of hydrophobic organic pollutants by titania pillared fluorine mica as a substrate specific photocatalyst. Appl. Catal. B Environ. 2005, 55, 141–148. [Google Scholar] [CrossRef]
- Guillard, C.; Disdier, J.; Monnet, C.; Dussaud, J.; Malato, S.; Blanco, J.; Maldonado, M.I.; Herrmann, J.M. Solar efficiency of a new deposited titaniaphotocatalyst: Cholorophenol, pesticide and dye removal applications. Appl. Catal. B Environ. 2003, 46, 319–332. [Google Scholar] [CrossRef]
- Rao, K.V.S.; Subrahmanyam, M.; Boule, P. Immobilized TiO2 photocatalyst during long-term use: Decrease of its activity. Appl. Catal. B Environ. 2003, 49, 239–249. [Google Scholar] [CrossRef]
- Zelenak, V.; Hornebecq, V.; Mornet, S.; Schaf, O.; Llewellyn, P. Mesoporous silica modified with titania: Structure and thermal stability. Chem. Mater. 2006, 18, 3184–3191. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Chan, K.Y. Synthesis of titania-silica mixed oxide mesoporous materials, characterization and photocatalytic properties. Appl. Catal. A Gen. 2005, 284, 193–198. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Lou, L. Photodegradtion of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 281–287. [Google Scholar] [CrossRef]
- Hirano, M.; Ota, K. Direct formation and photocatalytic performance of anatase (TiO2)/silica (SiO2) composite nanoparticles. J. Am. Ceram. Soc. 2004, 87, 1567–1570. [Google Scholar] [CrossRef]
- Ginger, D.S.; Zhang, H.; Mirkin, C.A. The evolution of dip-pen nanolithography. Angew. Chem. Int. Ed. 2004, 43, 30–45. [Google Scholar] [CrossRef]
- Park, H.; Lim, A.K.L.; Alivisatos, A.P.; Park, J.; McEuen, P.L. Fabrication of metallic electrodes with nanometer separation by electromigration. Appl. Phys. Lett. 1999, 75, 301–303. [Google Scholar] [CrossRef]
- Xu, L.; Vemula, S.C.; Jain, M.; Nam, S.K.; Donnelly, V.M.; Economou, D.J.; Ruchhoeft, P. Nanopantography: A new method for massively parallel nanopatterning over large areas. Nano Lett. 2005, 5, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Boncheva, M.; Whitesides, G.M. Theme article-making things by self-assembly. MRS Bull. 2005, 30, 736–742. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymer thermodynamics: Theory and experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Fasolka, M.J.; Mayes, A.M. Block copolymer thin films: Physics and applications. Annu. Rev. Mater. Res. 2001, 31, 323–355. [Google Scholar] [CrossRef]
- Hamley, W. The Physics of Block Copolymer; Oxford University Press: New York, NY, USA, 1999; pp. 58–85. [Google Scholar]
- Hashimoto, T.; Shilbayama, M.; Fujimura, M.; Kawai, H. Block Copolymers, Science and Technology; Harwood Academic: London, UK, 1993; pp. 63–98. [Google Scholar]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-induced self-assembly: Nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar]
- Yu, K.; Hurd, A.J.; Eisenberg, A.; Brinker, J. Synthesis of silica/polystyrene-block-poly(ethylene oxide) films with regular and reverse mesostructures of large characteristic length scales by solvent evaporation-induced self-assembly. Langmuir 2001, 17, 7961–7965. [Google Scholar] [CrossRef]
- Förster, S.; Antonietti, M. Amphiphilic block copolymers in structure-controlled nanomaterials hybrids. Adv. Mater. 1998, 10, 195–217. [Google Scholar] [CrossRef]
- Finnefrock, A.C.; Ulrich, R.; Gruner, S.M.; Wiesner, U. Metal oxide containing mesoporous silica with bicontinuous “Plumber’s Nightmare” morphology from a block copolymer hybrid mesophase. Angew. Chem. Int. Ed. 2001, 40, 1207–1211. [Google Scholar] [CrossRef]
- Simon, P.F.W.; Ulrich, R.; Spiess, H.W.; Wiesner, U. Block copolymer-ceramic hybrid materials from organically modified ceramic precursors. Chem. Mater. 2001, 13, 3464–3486. [Google Scholar] [CrossRef]
- Adachi, M.; Okumura, A.; Sivaniah, E.; Hashimoto, T. Incorporation of metal nanoparticles into a double gyroid network texture. Macromolecules 2006, 39, 7352–7357. [Google Scholar] [CrossRef]
- Lo, K.H.; Tseng, W.H.; Ho, R.M. In-situ formation of CdS nanoarrays by pore-filling nanoporous templates from degradable block copolymers. Macromolecules 2007, 40, 2621–2624. [Google Scholar] [CrossRef]
- Spontak, R.J.; Alexandridis, P. Advances in self-ordering macromolecules and nanostructure design. Curr. Opin. Colloid Interface Sci. 1999, 4, 140–146. [Google Scholar] [CrossRef]
- Li, X.; Peng, J.; Kang, J.H.; Choy, J.H.; Steinhart, M.; Knoll, W.; Kim, D.H. One step route to the fabrication of arrays of TiO2 nanobowls via a complementary block copolymer templating and sol-gel process. Soft Matter 2008, 4, 515–521. [Google Scholar]
- Kim, D.H.; Kim, S.H.; Lavery, K.; Russell, T.P. Inorganic nanodots from thin films of block copolymers. Nano Lett. 2004, 4, 1841–1844. [Google Scholar] [CrossRef]
- Kim, J.M.; Stucky, G.D. Synthesis of highly ordered mesoporous silica materials using sodium silicate and amphiphilic block copolymers. Chem. Commun. 2000. [Google Scholar] [CrossRef]
- Kim, D.H.; Sun, Z.; Russell, T.P.; Knoll, W.; Gutmann, J.S. Organic-inorganic nanohybridization by block copolymer thin films. Adv. Funct. Mater. 2000, 15, 1160–1164. [Google Scholar]
- Jang, Y.H.; Kochuveedu, S.T.; Cha, M.A.; Jang, Y.J.; Lee, J.Y.; Lee, J.; Lee, J.Y.; Kim, J.Y.; Ryu, D.Y.; Kim, D.H. Synthesis and photocatalytic properties of hierarchical metal nanoparticles/ZnO thin films hetero nanostructures assisted by diblock copolymer inverse micellar nanotemplates. J. Colloid Interface Sci. 2010, 345, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.; Moller, M.; Spatz, J.P. Block copolymer micelle nanolithography. Nanotechnology 2003, 14, 1153–1160. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, S.W.; Lee, S.C.; Lee, H.H.; Choung, S.J. Photocatalytic decomposition of gaseous formaldehyde using TiO2, SiO2-TiO2 and Pt-TiO2. Int. J. Photoenergy 2003, 5, 21–25. [Google Scholar]
- Chen, Y.; Wang, K.; Lou, L. Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J. Photochem. photobiol. A Chem. 2004, 163, 281–287. [Google Scholar]
Appendix



© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kannaiyan, D.; Kochuveedu, S.T.; Jang, Y.H.; Jang, Y.J.; Lee, J.Y.; Lee, J.; Lee, J.; Kim, J.; Kim, D.H. Enhanced Photophysical Properties of Nanopatterned Titania Nanodots/Nanowires upon Hybridization with Silica via Block Copolymer Templated Sol-Gel Process. Polymers 2010, 2, 490-504. https://doi.org/10.3390/polym2040490
Kannaiyan D, Kochuveedu ST, Jang YH, Jang YJ, Lee JY, Lee J, Lee J, Kim J, Kim DH. Enhanced Photophysical Properties of Nanopatterned Titania Nanodots/Nanowires upon Hybridization with Silica via Block Copolymer Templated Sol-Gel Process. Polymers. 2010; 2(4):490-504. https://doi.org/10.3390/polym2040490
Chicago/Turabian StyleKannaiyan, Dinakaran, Saji Thomas Kochuveedu, Yoon Hee Jang, Yu Jin Jang, Ji Yong Lee, Jieun Lee, Juyon Lee, Jooyong Kim, and Dong Ha Kim. 2010. "Enhanced Photophysical Properties of Nanopatterned Titania Nanodots/Nanowires upon Hybridization with Silica via Block Copolymer Templated Sol-Gel Process" Polymers 2, no. 4: 490-504. https://doi.org/10.3390/polym2040490
APA StyleKannaiyan, D., Kochuveedu, S. T., Jang, Y. H., Jang, Y. J., Lee, J. Y., Lee, J., Lee, J., Kim, J., & Kim, D. H. (2010). Enhanced Photophysical Properties of Nanopatterned Titania Nanodots/Nanowires upon Hybridization with Silica via Block Copolymer Templated Sol-Gel Process. Polymers, 2(4), 490-504. https://doi.org/10.3390/polym2040490