Periodate Oxidation of Methylcellulose: Characterization and Properties of Oxidized Derivatives
Abstract
:1. Introduction


2. Experimental Section
3. Results and Discussion
3.1. Introduction to Polysaccharide Oxidation
3.2. Characterization and Properties of Oxidized Methylcelluloses
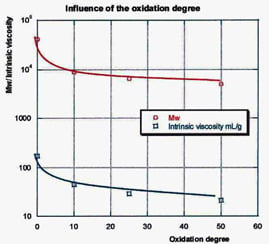
3.2.1. Characterization



3.2.2. Rheological Behavior of Methylcellulose.


3.2.3. Rheological Behavior of Oxidized Methylcelluloses
| G’ at 15 °C | G’’ at 15 °C | G’ at 70 °C | G’’ at 70 °C | G’/G’’ 70 °C | |
|---|---|---|---|---|---|
| Me cell Initial | 0.050 | 0.290 | 1500 | 47 | 32 |
| ME 10 | 0.021 | 0.114 | 115 | 10.5 | 11 |
| ME 25 | 0.032 | 0.126 | 25* | 2.6* | 10 |
| ME 50 | 0.028 | 0.100 | 24* | 2.8* | 8.5 |


3.3. Chemical Modification of Oxidized Methylcellulose

3.4. Crosslinking of Chitosan and Hyaluronan with Oxidized Methylcellulose
| Degree of oxidation | % nitrogen | Initial degree of swelling in water (g/g) | % w/w chitosan in the gel ** |
|---|---|---|---|
| ME 10 | 5.09 | 203 | 67 |
| ME 25 | 5.44 | 146 | 65 |
| ME 50 | 4.88 | 109 | 64 |
| Initial chitosan | 7.56 | --- |
| Medium | Degree of swelling (g/g)** |
|---|---|
| water | 35.5 |
| PBS buffer pH = 7.4 | 30.2 |
| Citrate-HCl buffer pH = 4 | 25.4 |
| 0.15M NaCl | 25.7 |

4. Conclusions
Acknowledgements
References
- Hirrien, M.; Desbrieres, J.; Rinaudo, M. Physical properties of methylcelluloses in relation with the conditions for cellulose modification. Carbohydr. Polym. 1996, 31, 243–252. [Google Scholar]
- Hirrien, M.; Chevillard, C.; Desbrieres, J.; Axelos, M.A.; Rinaudo, M. Thermogelation of methylcelluloses: new evidence for understanding the gelation mechanism. Polymer 1998, 39, 6251–6259. [Google Scholar] [CrossRef]
- Heymann, E. Sol-gel transformation.I.The inverse sol-gel transformation of methylcellulose in water. Trans. Faraday Soc. 1935, 31, 846–864. [Google Scholar] [CrossRef]
- Arisz, P.W.; Kauw, H.J.J.; Boon, J.J. Substitution distribution along the cellulose backbone in O-methylceluloses using GC and FAB-MS for monomer and oligomer analysis. Carbohydr. Res. 1995, 271, 1–14. [Google Scholar] [CrossRef]
- Vigouret, M.; Rinaudo, M.; Desbrieres, J. Thermogelation of methylcellulose in aqueous solutions. J. Chim. Phys. 1996, 93, 858–869. [Google Scholar]
- Desbrieres, J.; Hirrien, M.; Rinaudo, M. A calorimetric study of methylcellulose gelation. Carbohydr. Polym. 1998, 37, 145–152. [Google Scholar] [CrossRef]
- Desbrieres, J.; Hirrien, M.; Rinaudo, M. Relation between the conditions of modification and the properties of cellulose derivatives: thermogelation of methylcellulose. In Cellulose Derivatives: Modification, Characterization, and Nanostructures; Heinze, T., Glasser, W.G., Eds.; ACS Symp. Series 688; American Chemical Society: Washington, DC, USA, 1998; pp. 332–348. [Google Scholar]
- Hirrien, M. Comportement des Méthylcelluloses en Relation avec Leur Structure. Ph.D. Thesis, Grenoble University I, Grenoble, France, 1996. [Google Scholar]
- Yalpani, M.; Hall, L.D. Some chemical and analytical aspects of polysaccharide modifications. 3. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984, 17, 272–281. [Google Scholar]
- Hall, L.D.; Holme, K.R. Tailored rheology : chitosan derivatives with branched pendant sugar chain. J. Chem. Soc. Chem. Commun. 1986, 3, 217–218. [Google Scholar] [CrossRef]
- Rinaudo, M. New way to crosslink chitosan in aqueous solution. Eur. Polym. J. 2010, 46, 1537–1544. [Google Scholar] [CrossRef]
- Hutchins, R.O.; Natale, N.R. Cyanoborohydride. Utility and applications in organic synthesis. A review. Org Prep. Proced. Int. 1979, 11, 201–246. [Google Scholar] [CrossRef]
- Creuzet, C.; Kadi, S.; Rinaudo, M.; Auzely Velty, R. New associative systems based on alkylated hyaluronic acid. Synthesis and aqueous solution properties. Polymer 2006, 47, 2706–2713. [Google Scholar] [CrossRef]
- Rinaudo, M.; Auzely, R.; Kadi, S.; Bresin, A.; Kubik, E. New Derivatives of Hyaluronic Acid, Their Preparation Process and Their Uses. Patent No. WO2007059890, 2007. [Google Scholar]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Kidby, D.K.; Davidson, D.J. Convenient ferricyanide estimation of reducing sugars in the nanomole range. Anal. Biochem. 1973, 55, 321–325. [Google Scholar]
- Varma, A.J.; Kulkarni, M.P. Oxidation of cellulose under controlled conditions. Polym. Degrad. Stab. 2002, 77, 25–27. [Google Scholar] [CrossRef]
- Maekawa, E.; Koshijima, T. Properties of 2,3-dicarboxy cellulose combined with various metallic ions. J. Appl. Polym. Sci. 1984, 29, 2289–2297. [Google Scholar] [CrossRef]
- Sarymsakova, A.A.; Nadzhimutdinov, Sh.; Tashpulatov, Yu.T. Chemical transformations in the chains of cellulose dialdehydes and cellulose ethers. Chem. Nat. Compd. 1998, 34, 170–174. [Google Scholar] [CrossRef]
- Roberts, H.J. Nondegradative reactions of starch. In Starch: Chemistry and Technology; Whistler, R.L., Paschall, E.F., Eds.; Academic Press: London, UK, 1965; Volume 1, pp. 470–472. [Google Scholar]
- Whistler, R.L.; Daniel, J.R. Molecular structure of starch. In Starch: Chemistry and Technology, 2nd ed.; Whistler, R.L., BeMiller, J.N., Paschall, E.F., Eds.; Academic Press: San Diego, CA, USA, 1984; pp. 174–177. [Google Scholar]
- Gibbons, G.C. Periodate oxidation of water-soluble methylcellulose and its constituent methylglucoses. J. Textile Inst. Trans. 1956, 47, T511–T529. [Google Scholar] [CrossRef]
- Pendharkar, S.; Guo, J.X. Biodegradable Hemostatic Wound Dressings Containing Hemostatic Fabrics and Oxidized Polysaccharides. EP 1481694 A1 20041201, 2004. [Google Scholar]
- Pendharkar, S.M.; Wissing, W.K. Wound Dressing Containing Aldehyde-Modified Regenerated Polysaccharide. U.S. Pat. Appl. Publ. US 2004101547 A1 20040527, 2004. [Google Scholar]
- Wiseman, D.M.; Saferstein, L.; Wolf, S. Bioabsorbable Medical Devices from Oxidized Polysaccharides. U.S. Pat. Appl. Publ. US 2003073663 A1 20030417, 2003. [Google Scholar]
- Ye, Y.Q.; Ding, B.; Wang, K.; He, J.P.; Cui, J.J.; Jiang, B. Determination of the degree of substitution at secondary hydroxyls of hydroxypropyl guar gum by periodate oxidation. Gaodeng Xuexiao Huaxue Xuebao 2009, 30, 835–840. [Google Scholar]
- Lee, K.Y.; Bouhadir, K.H. Mooney DJ, Evaluation of chain stiffness of partially oxidized polyguluronate. Biomacromolecules 2002, 3, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Andresen, I.L.; Painter, T.; Smidsrod, O. Concerning the effect of periodate oxidation upon the intrinsic viscosity of alginate. Carbohydr. Res. 1977, 59, 563–566. [Google Scholar] [CrossRef]
- Cohen, J.D.; Chenault, H.K.; Schiffino, R.S. Preparation of Polysaccharide Dialdehydes Having High Purity. PCT Int. Appl. WO 2008133847 A1 20081106, 2008. [Google Scholar]
- Maia, J.; Ferreira, L.; Carvalho, R.; Ramos, M.A.; Gil, M.H. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer 2005, 46, 9604–9614. [Google Scholar] [CrossRef]
- Maeda, H.; Rambone, G.; Coviello, T.; Yuguchi, Y.; Urakawa, H.; Alhaique, F.; Kajiwara, K. Low-degree oxidized scleroglucan and its hydrogel. Int. J. Biol. Macromol. 2001, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Nadzhimutdinov, S.; Sarymsakov, A.A.; Usmanov, K.U. Regularities in the synthesis of cellulose dialdehyde and its ethers. Cellul. Chem. Technol. 1981, 15, 613–628. [Google Scholar]
- Perplies, E. Production of Temporarily Crosslinked Cellulose Ethers by Selective Oxidation of Hydroxyl Groups and Subsequent Crosslinking. PCT Int. Appl. WO 2003097700 A1 20031127, 2003. [Google Scholar]
- Sarymsakov, A.A.; Nadzhimutdinov, S.; Usmanov, K.U. Crosslinking of oxidized cellulose. Uzb. Khim. Zh. 1975, 19, 45–48. [Google Scholar]
- Sierakowski, M.R.; Milas, M.; Desbrieres, J.; Rinaudo, M. Specific modifications of galactomannans. Carbohydr. Polym. 2000, 42, 51–57. [Google Scholar] [CrossRef]
- Jiang, B.; Drouet, E.; Milas, M.; Rinaudo, M. Study on TEMPO-mediated selective oxidation of hyaluronan and the effects of salt on the reaction kinetics. Carbohydr. Res. 2000, 327, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Frollini, E.; Reed, W.F.; Milas, M.; Rinaudo, M. Polyelectrolytes from polysaccharides: selective oxidation of guar gum—A revisited reaction. Carbohydr. Polym. 1995, 27, 129–135. [Google Scholar] [CrossRef]
- Hall, L.D.; Yalpani, M. Synthesis of luminescent probe-sugar conjugates of either protected or unprotected sugars. Carbohydr. Res. 1980, 78, C4–C6. [Google Scholar] [CrossRef]
- Christiansen, K.A.; Balance, S.; Potthast, A.; Chistensen, B.E. An evaluation of tritium and fluorescence labelling combined with multi-detector SEC for the detection of carbonyl groups in polysaccharides. Carbohydr. Polym. 2009, 76, 196–205. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rinaudo, M. Periodate Oxidation of Methylcellulose: Characterization and Properties of Oxidized Derivatives. Polymers 2010, 2, 505-521. https://doi.org/10.3390/polym2040505
Rinaudo M. Periodate Oxidation of Methylcellulose: Characterization and Properties of Oxidized Derivatives. Polymers. 2010; 2(4):505-521. https://doi.org/10.3390/polym2040505
Chicago/Turabian StyleRinaudo, Marguerite. 2010. "Periodate Oxidation of Methylcellulose: Characterization and Properties of Oxidized Derivatives" Polymers 2, no. 4: 505-521. https://doi.org/10.3390/polym2040505
APA StyleRinaudo, M. (2010). Periodate Oxidation of Methylcellulose: Characterization and Properties of Oxidized Derivatives. Polymers, 2(4), 505-521. https://doi.org/10.3390/polym2040505