Partially Fluorinated Sulfonated Poly(ether amide) Fuel Cell Membranes: Influence of Chemical Structure on Membrane Properties
Abstract
: A series of fluorinated sulfonated poly (ether amide)s (SPAs) were synthesized for proton exchange membrane fuel cell applications. A polycondensation reaction of 4,4′-oxydianiline, 2-sulfoterephthalic acid monosodium salt, and tetrafluorophenylene dicarboxylic acids (terephthalic and isophthalic) or fluoroaliphatic dicarboxylic acids produced SPAs with sulfonation degrees of 80–90%. Controlling the feed ratio of the sulfonated and unsulfonated dicarboxylic acid monomers afforded random SPAs with ion exchange capacities between 1.7 and 2.2 meq/g and good solubility in polar aprotic solvents. Their structures were characterized using NMR and FT IR spectroscopies. Tough, flexible, and transparent films were obtained with dimethylsulfoxide using a solution casting method. Most SPA membranes with 90% sulfonation degree showed high proton conductivity (>100 mS/cm) at 80 °C and 100% relative humidity. Among them, two outstanding ionomers (ODA-STA-TPA-90 and ODA-STA-IPA-90) showed proton conductivity comparable to that of Nafion 117 between 40 and 80 °C. The influence of chemical structure on the membrane properties was systematically investigated by comparing the fluorinated polymers to their hydrogenated counterparts. The results suggest that the incorporation of fluorinated moieties in the polymer backbone of the membrane reduces water absorption. High molecular weight and the resulting physical entanglement of the polymers chains played a more important role in improving stability in water, however.1. Introduction
Environmental damage and related energy problems caused by burning fossil fuels have shifted research priorities to the discovery of alternative energy sources. Fuel cells can efficiently convert the chemical energy stored in fuels directly into electrical power without negative impacts on the environment, and this technology has been recognized as one of the most promising alternative and sustainable energy sources for automotive, portable, and stationary applications [1–6]. Many factors affect the performance of fuel cells. Among different types of fuel cells, proton exchange membrane fuel cells (PEMFCs) are considered the most promising type because of their high power density. Polymer electrolyte membranes (PEMs), a key component of PEMFCs, are the proton conductors in fuel cells and separate the hydrogen fuel from the oxidant. The harsh environments in which fuel cells operate—which include wide ranges of temperature, humidity, and pressure and the presence of oxidative species—set strict standards for effective PEM materials, including robust thermal, mechanical, and chemical stability as well as high proton conductivity.
During the last 40 years, perfluorosulfonic acid polymers such as Nafion have been used predominantly as PEMs in PEMFCs [7,8]. The perfluorinated structure provides good thermal and chemical stability and high proton conductivity. Nevertheless, inherent drawbacks such as difficult synthesis, high cost, and moderate operating temperature owing to low glass transition temperature have limited the practical application of these membranes in fuel cells. Thus, the development of low-cost, high-performance PEMs has been the focus of considerable research throughout the past decade [9–11]. A variety of advanced sulfonated polymers have been investigated as proton-conducting membranes, including polystyrene copolymers [12–14], poly(ether sulfone)s [15–18], poly(ether ketone)s [19–22], polyimides [23–25], and polybenzimidazoles [26,27]. Although these newer materials display good proton conductivity under certain conditions, none is close to being an ideal candidate for practical applications in PEMFCs.
In hydrocarbon-based PEMs, a high degree of sulfonation is generally required to achieve acceptable proton conductivity. A suitable amount of water sorption is also necessary for proton transport [28–30], but the resulting high ion exchange capacity (IEC) leads to considerable swelling of the membrane under hydrated conditions, which severely deteriorates its thermal and mechanical properties as well as overall fuel cell performance.
Sulfonated aromatic polyamides have been suggested as promising PEM materials because of their favorable mechanical properties and film-forming abilities [31–33]. Despite these potential advantages, however, their application in fuel cell membranes is hampered by their instability toward hydrolysis and heat. Introducing flexible linkages, separating the sulfonic acid group from the diamine parts, and using aliphatic diamines could improve the stability of these materials, but research on more stable membranes would remain highly desirable [34].
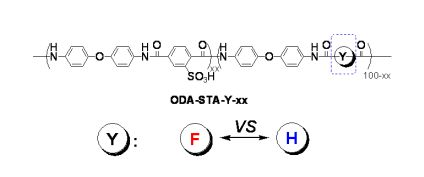
Fluorine-containing polymers and materials display good thermal and chemical stability and high hydrophobicity—properties that make them useful in a wide variety of applications [35,36]. We have previously reported our discovery that water adsorption in various sulfonated poly(ether amide)s (SPA) can be reduced by incorporating bulky aromatic diamine structures [37]. SPAs with an IEC less than 1.80 mequiv/g showed good stability in water and high proton conductivity comparable to that of Nafion. Higher sulfonation level in SPAs would improve proton conductivity further. Unfortunately, SPAs with IEC > 1.80 were found to have a limited stability in water, causing dissolution of the membranes. These results lead us to investigate ionic polymers with different structures (e.g., fluorinated vs. hydrogenated groups) and their structure-related properties of these membranes. Herein we report our systematic comparison of the synthesis and properties of various aromatic poly(ether amide)s and their fluorinated analogs. We focus specifically on the influence of the chemical structure and molecular weight of the ionomers on membrane properties, including water sorption, stability, and conductivity.
2. Results and Discussion
2.1. Polymer Synthesis and NMR Characterization
The preparation of various SPAs containing different fluorine segments and their hydrogen-containing counterparts is shown in Scheme 1. Using our previously reported conditions [37], we reacted a diamine monomer (4,4′-oxydianiline, ODA) with a sulfonated dicarboxylic acid (2-sulfoterephthalic acid monosodium salt, STA) and unsulfonated dicarboxylic acids (the box in Scheme 1 gives structural details) in a one-pot polycondensation in the presence of pyridine and triphenylphosphite to produce a series of SPAs in high yields. To obtain a variety of polymer structures for comparison, we used both aryl and aliphatic dicarboxylic acids in the polycondensation. Incorporating aliphatic moieties in the polymer main chains provides flexibility and enhanced solubility. We obtained SPAs in sodium salt form that possessed good solubility in dimethylsulfoxide (DMSO), and casting from a DMSO solution was used to procure flexible, transparent, yellowish-brown membranes.
The prepared SPAs were characterized using 1H and 19F NMR spectroscopies. As shown in Figure 1, both fluorine- and hydrogen-containing SPAs showed two resonances at 10.5 and 11.3 ppm, which were assigned as the two amide hydrogens of STA owing to the electron-withdrawing effect of the sulfonate group. For ODA-STA-TPA-90 and ODA-STA-TPA-80 (TPA = terephthalic acid), a resonance appeared at 10.4 ppm that was attributed to the amide hydrogen in the TPA unit. This peak was more intense in the spectrum of ODA-STA-TPA-80 compared to that of ODA-STA-TPA-90 because of its slightly higher TPA concentration. Based on the integral ratio of amide hydrogen resonances from the STA and TPA parts in the polymers, the calculated degrees of sulfonation were 89% and 78%, respectively, which matched well with values of the sulfonated monomer feed ratio. Interestingly, the peak for the amide hydrogens of the tetrafluoroterephthalic acid (TFTPA) unit appeared at 8.64 ppm, a shift of nearly 2 ppm upfield from that of TPA. When fluoroalkyl dicarboxylic acids (i.e., hexafluoroglutaric acid [HFGA], perfluorosebacic acid [PFSEA], perfluorosuberic acid [PFSUA], shown in Scheme 1) were incorporated into the condensation polymerization, however, their amide hydrogens showed a deshielded resonance at 11.3 ppm versus 9.8 ppm in the hydrogenated alkyl compounds (Figure 2).
The successful incorporation of fluorine moieties into the fluorinated SPA backbones was also confirmed using 19F NMR spectroscopy (Figure 3). Although before polymerization, TFTPA and tetrafluoroisophtalic acid (TFIPA) have one and three kinds of fluorine, respectively, additional resonances were observed after polymerization because the fluoride groups in the polymers have asymmetrical chemical environments presumably caused by the formation of hydrogen bonding with amide hydrogens. Thus, the fluorine atoms of ODA-STA-TFIPA displayed four resonances with equal integral intensity at ‒116, ‒130, ‒135, and ‒164 ppm (Figure 3(a)). For ODA-STA-TFTPA, two peaks at ‒138 and ‒142 ppm appeared in a 1:1 ratio. The 19F NMR spectra of fluoroalkylated SPAs also matched well with their chemical structures (Figure 3(c,d)).
2.2. Properties of Sulfonated Poly(ether amide)s
Observations of the influence of chemical structure on the reactivity of the polymerization revealed the following: terephthalic acid monomers (TPA and TFTPA) showed enhanced reactivity compared with isophthalic acid monomers (IPA and TFIPA) with both fluorinated and unfluorinated dicarboxylic acids. Polymerization with TPA was stopped after 0.5 h owing to the high viscosity of the reaction medium. Compared with their unfluorinated hydrocarbon counterparts, the fluorinated dicarboxylic acids showed lower reactivities and reaction rates; therefore, a longer reaction time (i.e., 24 h) was required for the polycondensation of fluorinated dicarboxylic acids (TFTPA, TFIPA, HFGA, PFSEA, PFSUA). High yields (>97%) were obtained in all the reactions, and off-white fibrous polymers were obtained after precipitation twice from methanol.
A polymer must have a high molecular weight to maintain good mechanical properties and stability in fuel cell membrane applications. The intrinsic viscosities of the SPAs were measured in a 0.1 M sodium iodide solution of DMSO at 30 °C. At a 90% degree of sulfonation, all polymers showed higher viscosity than they did at 80%. Owing to the lower reactivity of the fluorinated dicarboxylic acids in the polycondensation reaction, fluorinated SPAs had lower molecular weights than their hydrogenated counterparts. Thus, their viscosity values were typically about half or less those of hydrogenated SPAs (Table 1). The lower reactivity of isophthalic acid monomers also resulted in lower viscosity values than those of terephthalic acid derivatives (Table 1, entries 1 and 2 vs. 3 and 4).
Flexible and transparent membranes were easily cast from a DMSO solution using sodium salt forms of the SPAs. After the membranes were acidified in 1 M sulfuric acid solution and rinsed with deionized water, their water adsorption properties were measured. IECs were measured from titration and compared with those of calculated values based on the molar feed ratio of STA. As shown in Table 1, the experimental results correspond well with the calculated values. For membranes with similar structure, the higher the IEC, the greater the water uptake of the membrane was observed. Thus, ionomers with a 90% degree of sulfonation had higher water uptake values than those with a degree of sulfonation of 80%. Furthermore, the water uptake in fluorinated membranes was slightly lower than that of their hydrogenated counterparts. For example, the water uptake of ODA-STA-TPA-90 is 65%, which is about 10% higher than ODA-STA-TFTPA-90. This lower water uptake of fluorinated ionomers is also evident when we compare the lambda values (number of water molecules per SO3H group) of the membranes (see Table 1). Overall, these results suggest that the presence of fluorinated segments reduces water adsorption of the membranes, possibly owing to their relatively lower IECs and/or greater hydrophobicity.
The results of hydrolytic stability studies of the SPA membranes are shown in Table 2. The membranes displayed low hydrolytic stability, and some of them were dissolved in less than 1 h under the testing conditions (i.e., deionized water at 80 °C). To investigate whether the poor stability in water is a result of degradation of amide bonds in the polymer chains or dissolution of the polymers due to high sulfonation degree, we dissolved ODA-STA-TFIPA-90 and ODA-STA-TFIPA-80 (in –SO3H form) in hot water and compared their viscosity values (in DMSO) and 1H NMR spectra with those of untreated polymers. The change in viscosity after dissolving in water was negligible. The treated ionomer polymers also showed almost identical 1H NMR spectra with unaffected NH resonances of amide bonds. These results indicate that poor stability of the ionomers in water is not caused by acidic hydrolysis of the amide bonds of the polymer chain at least under the condition but simple dissolution of the highly sulfonated polymers (IEC > 1.8). Despite their lower water uptake, the fluorinated SPA membranes were surprisingly less stable than the corresponding hydrogenated membranes, possibly because of their lower molecular weights and reduced physical entanglement.
2.3. Proton Conductivity
Table 3 and Figure 4 show proton conductivity data of SPA membranes at different temperatures under 100% relative humidity. Proton conductivities of these ionomers are strongly dependent on IEC and temperature: membranes with higher IECs tend to have higher proton conductivity, and proton conductivity increases with temperature. Most of the SPA membranes displayed lower proton conductivities than that of Nafion 117 at low temperatures, but ODA-STA-TPA-90 and ODA-STA-IPA-90 exhibited proton conductivity comparable to that of Nafion 117 at temperatures between 40 and 80 °C. Among examined SPA membranes, ODA-STA-GA-90 provided the highest conductivity of 165.6 mS/cm at 80 °C—a value higher than that of Nafion. Unfortunately, its conductivity dropped dramatically with decreasing temperature. The typical heavy dependence of hydrocarbon-based PEM's proton conductivity on temperature can be attributed to the lower acidity of the sulfonic acid group and the less pronounced phase-separated morphology of the randomly sulfonated ionomers compared with those of Nafion. Nevertheless, all SPA membranes prepared in this study exhibited sufficiently high proton conductivity.
2.4. Thermal Properties
SPA membranes in acid form were subjected to thermogravimetric analysis to evaluate their thermal decomposition properties. A slight weight loss in the membranes below 150 °C was due to the evaporation of water and other solvent residues. For aromatic SPAs, the next weight decrease occurred around 250 °C, which was ascribed to desulfonation of the ionomers (Figure 5(a)). The thermogravimetric analysis showed that most of the aromatic SPA membranes retained approximately 70% of their weights even at high temperatures up to 530 °C.
In the case of aliphatic SPAs, decomposition of the aliphatic chains started around 180 °C (Figure 5(b)). Due to higher bond dissociation energy of C–F bond than C–H bond, the fluorinated aliphatic SPAs showed better thermal stability than the non-fluorinated counterparts.
3. Experimental Section
3.1. Materials and Methods
4,4′-Oxydianiline (ODA), lithium chloride (>99%), 2-sulfoterephthalic acid monosodium salt (STA, >98%), tetrafluoroterephthalic acid (TFTPA, 97%), tetrafluoroisophthalic acid (TFIPA, 98%), terephthalic acid (TPA, 98%), isophthalic acid (IPA, 99%), glutaric acid (GA, 99%), suberic acid (SUA, 98%), sebacic acid (SEA, 99%), hexafluoroglutaric acid (HFGA, 98%), perfluorosuberic acid (PFSUA, 98%), perfluorsebacic acid (PFSEA, 96%), pyridine (99%), 1-methyl-2-pyrrolidinone (NMP, extra dry with molecular sieve) were purchased from commercial vendors and used as received. Triphenylphosphite (TPP) was purified by distillation under reduced pressure and stored in a nitrogen-filled glove box until its use.
3.2. Synthesis of Sulfonated Poly(ether amide)s
In a nitrogen-filled glove box, ODA (0.40 g, 2.0 mmol), different ratio of STA and hydrogenated or fluorinated dicarboxylic acid (2.0 mmol in total), lithium chloride (0.41 g, 9.6 mmol), calcium chloride (0.60 g, 5.4 mmol), TPP (1.68 g, 5.40 mmol), and NMP (10 mL) were added in sequence to a 25 mL vial and capped with a Teflon-lined septum. The vial was removed from the glove box, pyridine (2.6 mL) was added to the vial via a syringe. The vial was placed in 115 °C oil bath and stirred for given time shown in Table 1. After cooling to room temperature, the reaction solution was added dropwise to methanol to precipitate polymer. After filtering, the recovered polymer was dissolved in DMSO (9 mL) and reprecipitated by adding methanol (80 mL). The polymer was dried at 80 °C overnight under vacuum.
3.3. Membrane Preparation of Sulfonated Poly(ether amide)s
The sulfonated poly (ether amide) in sodium salt form (–SO3Na) (0.60–0.70 g) was dissolved in DMSO (approximately 5 mL) with gentle heating and the resulting clear solution was cast on a clean glass plate. After dried overnight at 50 °C with a positive air flow, the membrane was further dried at 80 °C for 6 h under reduced pressure. The membrane was peeled off from the glass plate by immersing in deionized water and rinsed with water to remove any residual solvent. Acidification was conducted by immersing the membrane in 1 M H2SO4 solution for 3 days, during which period the solution was changed daily. The membrane was then soaked in deionized water for 12 h to remove any excess acid.
3.4. Measurements and Characterization
1H and 19F NMR spectra of the polymers in sodium salt form were obtained using a 400 MHz and 376 MHz Varian NMR spectrometer at room temperature. The NMR samples were prepared by dissolving polymer in DMSO-d6. Chemical shifts were referred to DMSO residue (2.50 ppm) for 1H NMR and CFCl3 for 19F NMR. The intrinsic viscosities of sulfonated poly(ether amide)s in sodium salt form were measured using an Ubbelohde viscometer in a 0.1 M NaI/DMSO solution at 30 °C. Thermo gravimetric analysis (TGA) of the sulfonated polymers was performed under a nitrogen flow at a rate of 40 mL/min. The TGA data was collected between 30 °C and 560 °C at a heating rate of 10 °C/min using a Netzsch STA 440 TGA/DSC instrument.
The calculated IECs of the sulfonated polymers were estimated from the feeding molar ratio of unsulfonated dicarboxylic acid to STA. The experimental IECs of the membranes were determined from a typical titration method. The dried membrane in acid form was equilibrated with 60 mL of 2 M NaCl solution for 3 days for proton exchange with sodium cation. The amount of released protons from the membrane to the solution was titrated with a 0.025 M NaOH solution using phenolphthalein solution as indicator. The degree of sulfonation was estimated from molar feed ratio of unsulfonated dicarboxylic acid to STA and confirmed by 1H NMR spectra.
For water uptake (WU) measurement, membranes in acid form were immersed in deionized water at room temperature for 24 h. The membrane was removed from water, after wiping off surface water with a tissue, it was weighed on a microbalance to give WWet. The membrane was then dried at 80 °C under reduced pressure for 12 h and weighed to give WDry. The water uptake was calculated according to the equation of (WWet – WDry)/WDry × 100%.
The hydrolytic stability of SPA membranes in sulfonic acid form was tested by soaking the films in deionized water at 80 °C. The hydrolytic stability was evaluated by measuring the elapsed time for the membrane to completely dissolve into the water solution.
The SPA membranes in acid form were immersed in deionized water for at least 24 h before the measurement of proton conductivity. The proton conductivity was measured using a four-electrode method with a BT-512 membrane conductivity test system (BekkTech LLC). Proton conductivity measurement was conducted by changing the temperature from 40 to 80 °C under 100% relative humidity. The conductivity was calculated using the equation below:
4. Conclusion
We have prepared a series of SPA polymers through polycondensation of ODA with STA and various dicarboxylic acid monomers (fluorinated, unfluorinated, aromatic, and aliphatic). The structures of the polymers were thoroughly characterized and confirmed using NMR spectroscopy. Systematic comparisons of both the fluorinated structures and their hydrogenated counterparts as well as the aryl and aliphatic segments of the sulfonated polymers were made. Although the proton conductivity of the random sulfonated polymers showed heavy dependence on temperature, it was sufficiently high in all of the polymers at 80 °C. The incorporation of fluorinated segments led to reduced water adsorption—presumably because of the increased hydrophobicity of the fluorine group—and enhanced thermal stability. However, the reduced reactivity of fluorinated dicarboxylic acids in the polycondensations also induced lower molecular weight in fluorinated SPA polymers, as indicated by lower viscosity.






| Entry | Ionomer | Time (h) | Inherent viscosityb | Water uptake (%)c | λ(H2O/SO3H) | IEC(meq/g) | |
|---|---|---|---|---|---|---|---|
| Exp.d | Cacl.e | ||||||
| 1 | ODA-STA-TPA-90 | 0.5 | 1.83 | 65 | 16.3 | 2.22 | 2.24 |
| 2 | ODA-STA-TPA-80 | 0.5 | 1.78 | 53 | 14.4 | 2.05 | 2.03 |
| 3 | ODA-STA-IPA-90 | 14 | 0.79 | 67 | 17.3 | 2.15 | 2.24 |
| 4 | ODA-STA-IPA-80 | 14 | 0.68 | 50 | 14.0 | 1.98 | 2.03 |
| 5 | ODA-STA-TFTPA-90 | 24 | 0.97 | 55 | 14.6 | 2.10 | 2.20 |
| 6 | ODA-STA-TFTPA-80 | 24 | 0.81 | 48 | 13.7 | 1.94 | 1.96 |
| 7 | ODA-STA-TFIPA-90 | 24 | 0.64 | 50 | 13.0 | 2.13 | 2.20 |
| 8 | ODA-STA-TFIPA-80 | 24 | 0.54 | 48 | 14.0 | 1.90 | 1.96 |
| 9 | ODA-STA-GA-90 | 2 | 1.93 | 42 | 11.1 | 2.01 | 2.26 |
| 10 | ODA-STA-GA-80 | 2 | 1.48 | 40 | 13.0 | 1.71 | 2.06 |
| 11 | ODA-STA-SEA-90 | 2 | 0.98 | 42 | 12.6 | 1.85 | 2.23 |
| 12 | ODA-STA-SEA-80 | 6 | 1.97 | 38 | 12.1 | 1.75 | 2.02 |
| 13 | ODA-STA-SUA-90 | 16.5 | 0.91 | 38 | 11.1 | 1.90 | 2.22 |
| 14 | ODA-STA-SUA-80 | 16.5 | 1.31 | 35 | 10.6 | 1.84 | 1.99 |
| 15 | ODA-STA-HFGA-90 | 24 | 0.89 | 36 | 9.9 | 2.02 | 2.20 |
| 16 | ODA-STA-HFGA-80 | 24 | 0.53 | 33 | 10.5 | 1.75 | 1.96 |
| 17 | ODA-STA-PFSEA-90 | 24 | 0.82 | 34 | 9.9 | 1.91 | 2.12 |
| 18 | ODA-STA-PFSEA-80 | 24 | 0.64 | 32 | 10.0 | 1.78 | 1.82 |
| 19 | ODA-STA-PFSUA-90 | 24 | 0.60 | 37 | 10.8 | 1.90 | 2.07 |
| 20 | ODA-STA-PFSUA-80 | 24 | 0.53 | 33 | 11.1 | 1.65 | 1.74 |
aAll the reactions were carried out with 0.40 g of 4,4′-oxydianiline (ODA) under standard conditions (see experimental section for details).bMeasured with –SO3−Na+ form of ionomer (0.5 mg/mL) in a 0.1 M NaI/DMSO solution) using an Ubbelohde viscometer at 30 °C.cWater uptake (%) = (Wwet ‒ Wdry)/Wdry × 100, where Wwet and Wdry are the weights of the dry and wet membranes.dMeasured from titration.eCalculated from the molar feed ratio of STA and dicarboxylic acid.
| Ionomer | Time a | |
|---|---|---|
| ODA-STA-TPA-90 | 5 h | |
| ODA-STA-TFTPA-90 | 10 minutes | |
| ODA-STA-TPA-80 | >24 h | |
| ODA-STA-TFTPA-80 | 38 minutes | |
| ODA-STA-IPA-90 | 5 minutes | |
| ODA-STA-TFIPA-90 | 5 minutes | |
| ODA-STA-IPA-80 | 27 minutes | |
| ODA-STA-TFIPA-80 | 14 minutes | |
aThe elapsed time for a membrane to dissolve completely in deionized water at 80 °C.
| Ionomer | IECb | Water Uptake | Proton conductivity (mS/cm) | ||
|---|---|---|---|---|---|
| 40 °C | 60 °C | 80 °C | |||
| ODA-STA-TPA-90 | 2.22 | 65 | 76.0 | 98.2 | 158.2 |
| ODA-STA-TPA-80 | 2.05 | 53 | 57.8 | 81.6 | 129.5 |
| ODA-STA-IPA-90 | 2.15 | 67 | 78.7 | 98.8 | 142.3 |
| ODA-STA-TFTPA-90 | 2.10 | 55 | 69.3 | 83.1 | 140.4 |
| ODA-STA-TFTPA-80 | 1.94 | 48 | 43.2 | 68.9 | 117.0 |
| ODA-STA-TFIPA-90 | 2.13 | 50 | 45.0 | 67.1 | 109.6 |
| ODA-STA-GA-90 | 2.01 | 42 | 50.3 | 99.5 | 165.6 |
| ODA-STA-GA-80 | 1.71 | 40 | 45.6 | 70.6 | 103.6 |
| ODA-STA-SEA-90 | 1.85 | 42 | 35.9 | 71.7 | 99.7 |
| ODA-STA-SEA-80 | 1.75 | 38 | 19.7 | 35.6 | 40.8 |
| ODA-STA-SUA-90 | 1.90 | 38 | 34.7 | 83.1 | 126.2 |
| ODA-STA-SUA-80 | 1.84 | 35 | 36.9 | 45.5 | 77.6 |
| ODA-STA-HFGA-90 | 2.02 | 36 | 52.0 | 78.3 | 114.5 |
| ODA-STA-HFGA-80 | 1.75 | 33 | 27.7 | 57.6 | 93.6 |
| ODA-STA-PFSEA-90 | 1.91 | 34 | 55.4 | 72.4 | 125.4 |
| ODA-STA-PFSEA-80 | 1.78 | 32 | 45.9 | 64.8 | 103.4 |
| ODA-STA-PFSUA-90 | 1.90 | 37 | 45.1 | 68.5 | 110.6 |
| ODA-STA-PFSUA-80 | 1.65 | 33 | 28.7 | 48.1 | 90.8 |
| Nafion 117 | 0.90 | 19 | 80.2 | 103.7 | 143.2 |
aProton conductivity was measured at 100% relative humidity at temperatures of 40–80 °C.bIon exchange capacity (IEC) in meq/g measured from titration.
Acknowledgments
We thank the National Science Foundation (CAREER DMR-0747667) and Nevada Renewable Energy Consortium for generous support of this project.
References
- Cleghorn, C.; Ren, X.; Springer, T.E.; Wilson, M.S.; Zawodzinski, C.; Zawodzinski, T.A.; Gottesfeld, S. PEM Fuel Cells for Transportation and Stationary Power Generation Applications. Int. J. Hydrogen Energ. 1997, 22, 1137–1142. [Google Scholar]
- Kordesch, K.; Simader, G.R. Fuel Cells and Their Applications; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Steele, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar]
- Winter, M.; Brodd, R.J. What are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar]
- Mehta, V.; Cooper, J.S. Review and Analysis of PEM Fuel Cell Design and Manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar]
- Eisenberg, A.; Yeager, H.L. Perfluorinated Ionomer Membranes; ACS Symposium Series 180; American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Nakajima, T.; Groult, H. Fluorinated Materials for Energy Conversion; Elsevier: Oxford, UK, 2005. [Google Scholar]
- Rikukawa, M.; Sanui, K. Proton-Conducting Polymer Electrolyte Membranes Based on Hydrocarbon Polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar]
- Hickner, M.; Ghassemi, H.; Kim, Y.S.; Einsla, B.; McGrath, J.E. Alternative Polymer Systems for Proton Exchange Membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar]
- Miyatake, K.; Watanabe, M. Emerging Membrane Materials for High Temperature Polymer Electrolyte Fuel Cells: Durable Hydrocarbon Ionomers. J. Mater. Chem. 2006, 16, 4465–4467. [Google Scholar]
- Kim, J.; Kim, B.; Jung, B. Proton Conductivities and Methanol Permeabilities of Membranes Made from Partially Sulfonated Polystyrene-block-Poly(ethylene-ran-butylene)-block-Polystyrene Copolymers. J. Membr. Sci. 2002, 207, 129–137. [Google Scholar]
- Ding, J.; Chuy, C.; Holdcroft, S. Enhanced Conductivity in Morphologically Controlled Proton Exchange Membranes: Synthesis of Macromonomers by SFRP and Their Incorporation into Graft Polymers. Macromolecules 2002, 35, 1348–1355. [Google Scholar]
- Serpico, J.M.; Ehrenberg, S.G.; Fontanella, J.J.; Jiao, X.; Perahia, D.; McGrady, K.A.; Sanders, E.H.; Kellogg, G.E.; Wnek, G.E. Transport and Structural Studies of Sulfonated Styrene-Ethylene Copolymer Membranes. Macromolecules 2002, 35, 5916–5921. [Google Scholar]
- Kim, D.S.; Robertson, G.P.; Guiver, M.D. Comb-Shaped Poly(arylene ether sulfone)s as Proton Exchange Membranes. Macromolecules 2008, 41, 2126–2134. [Google Scholar]
- Hou, J.; Li, J.; Madsen, L.A. Anisotropy and Transport in Poly(arylene ether sulfone) Hydrophilic-Hydrophobic Block Copolymers. Macromolecules 2010, 43, 347–353. [Google Scholar]
- Ghassemi, H.; Ndip, G.; McGrath, J.E. New Multiblock Copolymers of Sulfonated Poly(4′-phenyl-2,5-benzophenone) and Poly(arylene ether sulfone) for Proton Exchange Membranes. II. Polymer 2004, 45, 5855–5862. [Google Scholar]
- Kim, Y.S.; Einsla, B.; Sankir, M.; Harrison, W.; Pivovar, B.S. Structure-Property-Performance Relationships of Sulfonated Poly(arylene ether sulfone)s as a Polymer Electrolyte for Fuel Cell Applications. Polymer 2006, 47, 4026–4035. [Google Scholar]
- Kreuer, K.D. On the Development of Proton Conducting Polymer Membranes for Hydrogen and Methanol Fuel Cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymer Proton Conducting Membranes for Medium Temperature Fuel Cells (110–160 °C). J. Membr. Sci. 2001, 185, 73–81. [Google Scholar]
- Zaidi, S.M.J.; Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Proton Conducting Composite Membranes from Polyether Ether Ketone and Heteropolyacids for Fuel Cell Applications. J. Membr. Sci. 2000, 173, 17–34. [Google Scholar]
- Liu, B.; Robertson, G.P.; Guiver, M.D.; Sun, Y.M.; Liu, Y.L.; Lai, J.Y.; Mikhailenko, S.; Kaliaguine, S. Sulfonated Poly (aryl ether ether ketone ketone)s Containing Fluorinated Moieties as Proton Exchange Membrane Materials. J. Polym. Sci. B Polym. Phys. 2006, 44, 2299–2310. [Google Scholar]
- Miyatake, K.; Zhou, H.; Uchida, H.; Watanabe, M. Highly Proton Conductive Polyimide Electrolytes Containing Fluorenyl Groups. Chem. Commun. 2003, 38, 368–369. [Google Scholar]
- Einsla, B.R.; Kim, Y.S.; Hickner, M.A.; Hong, Y.T.; Hill, M.L.; Pivovar, B.S.; McGrath, J.E. Sulfonated Naphthalene Dianhydride Based Polyimide Copolymers for Proton-Exchange-Membrane Fuel Cells II. Membrane Properties and Fuel Cell Performance. J. Membr. Sci. 2005, 255, 141–148. [Google Scholar]
- Einsla, B.R.; Hong, Y.T.; Kim, Y.S.; Wang, F.; Gunduz, N.; McGrath, J.E. Sulfonated Naphthalene Dianhydride Based Polyimide Copolymers for Proton-Exchange-Membrane Fuel Cells. I. Monomer and Copolymer Synthesis. J. Polym. Sci. A Polym. Chem. 2004, 42, 862–874. [Google Scholar]
- Jones, D.J.; Rozière, J. Recent Advances in the Functionalization of Polybenzimidazole and Polyetherketone for Fuel Cell Applications. J. Membr. Sci. 2001, 185, 41–58. [Google Scholar]
- Yu, S.; Benicewicz, B.C. Synthesis and Properties of Functionalized Polybenzimidazoles for High-Temperature PEMFCs. Macromolecules 2009, 42, 8640–8648. [Google Scholar]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in Proton Conductors for Fuel-Cell Applications: Simulations, Elementary Reactions, and Phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar]
- Kreuer, K.D. On the Complexity of Proton Conduction Phenomena. Solid State Ionics 2000, 136-137, 149–160. [Google Scholar]
- Zawodzinski, T.A.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of Water Diffusion Coefficients in Perfluorosulfonate Ionomeric Membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar]
- Taeger, A.; Vogel, C.; Lehmann, D.; Jehnichen, D.; Komber, H.; Meier-Haack, J.; Ochoa, N.A.; Nunes, S.P.; Peinemann, K.V. Ion Exchange Membranes Derived from Sulfonated Polyaramides. React. Funct. Polym. 2003, 57, 77–92. [Google Scholar]
- Every, H.A.; Mendes, E.; Picken, S.J. Ordered Structures in Proton Conducting Membranes from Supramolecular Liquid Crystal Polymers. J. Phys. Chem. B 2006, 110, 23729–23735. [Google Scholar]
- Every, H.A.; Janssen, G.J.M.; Sitters, E.F.; Mendes, E.; Picken, S.J. Performance Analysis of Sulfonated PPTA Polymers as Potential Fuel Cell Membranes. J. Power Sources 2006, 162, 380–387. [Google Scholar]
- Wang, L.; Yi, B.L.; Zhang, H.M.; Xing, D.M. Characteristics of Polyethersulfone/Sulfonated Polyimide Blend Membrane for Proton Exchange Membrane Fuel Cell. J. Phys. Chem. B 2008, 112, 4270–4275. [Google Scholar]
- Souzy, R.; Ameduri, B.; Boutevin, B.; Capron, P.; Marsacq, D.; Gelbel, G. Proton-Conducting Polymer Electrolyte Membranes Based on Fluoropolymers Incorporating Perfluorovinyl Ether Sulfonic Acids and Fluoroalkenes: Synthesis and Characterization. Fuel Cells 2005, 5, 383–397. [Google Scholar]
- Amedrui, B.; Boutevin, B. Well-Architectual Fluoropolymer: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherland, 2004. [Google Scholar]
- Jo, T.S.; Ozawa, C.H.; Eagar, B.R.; Brownell, L.V.; Han, D.; Bae, C. Synthesis of Sulfonated Aromatic Poly (ether amides) and Their Applications to Proton Exchange Membrane Fuel Cells. J. Polym. Sci. A Polym. Chem. 2009, 47, 485–496. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, Y.; Lee, Y.-B.; Bae, C. Partially Fluorinated Sulfonated Poly(ether amide) Fuel Cell Membranes: Influence of Chemical Structure on Membrane Properties. Polymers 2011, 3, 222-235. https://doi.org/10.3390/polym3010222
Chang Y, Lee Y-B, Bae C. Partially Fluorinated Sulfonated Poly(ether amide) Fuel Cell Membranes: Influence of Chemical Structure on Membrane Properties. Polymers. 2011; 3(1):222-235. https://doi.org/10.3390/polym3010222
Chicago/Turabian StyleChang, Ying, Yeong-Beom Lee, and Chulsung Bae. 2011. "Partially Fluorinated Sulfonated Poly(ether amide) Fuel Cell Membranes: Influence of Chemical Structure on Membrane Properties" Polymers 3, no. 1: 222-235. https://doi.org/10.3390/polym3010222
APA StyleChang, Y., Lee, Y. -B., & Bae, C. (2011). Partially Fluorinated Sulfonated Poly(ether amide) Fuel Cell Membranes: Influence of Chemical Structure on Membrane Properties. Polymers, 3(1), 222-235. https://doi.org/10.3390/polym3010222