Polarized Emission of Wholly Aromatic Bio-Based Copolyesters of a Liquid Crystalline Nature
Abstract
: A novel thermotropic liquid crystalline polymers poly{3-benzylidene amino-4-hydroxybenzoic acid (3,4-BAHBA)-co-trans-4-hydroxycinnamic acid (4HCA: trans-coumaric acid)} (Poly(3,4-BAHBA-co-4HCA)), was synthesized by the thermal polycondensation of 4HCA and 3,4-BAHBA, which was synthesized by a reaction of 3-amino-4-hydroxybenzoic acid (3,4-AHBA) with benzaldehyde. When the 4HCA compositions of Poly(3,4-BAHBA-co-4HCA)s were above 55 mol%, the copolymers showed a nematic, liquid crystalline phase. Differential scanning calorimetry (DSC) measurements of the copolymers showed a high glass transition temperature of more than 100 °C, sufficient for use in engineering plastics. Furthermore, the copolymers showed photoluminescence in an N-methylpyrrolidone (NMP) solution under ultraviolet (UV) light with a wavelength of 365 nm. Oriented film of Poly(3,4-BAHBA-co-4HCA) with a 4HCA composition of 75 mol% emitted polarized light, which was confirmed by fluorescent spectroscopy equipped with parallel and crossed polarizers.1. Introduction
Liquid crystalline (LC) polymers are easily oriented at the molecular level to create materials with ultrahigh-strength, a high modulus, and other orientation-related functions [1,2]. For example, poly(p-phenylene terephthalamide) (Kevlar™) and poly(p-phenylene-2,6-benzobisoxazole) (Zylon™), which have a high proportion of rigid aromatic moieties, showed lyotropic liquid crystals that could be induced to prepare highly-oriented fibers [3,4]. They are widely used as electrical insulation, protective clothing, straps, cables, and aerospace materials. On the other hand, wholly aromatic LC polymers have rarely been synthesized from bio-derived monomers, but have gained recognition in the field of sustainability science for many natural LC polymers such as DNA [5,6], RNA [7], hemoglobin S (HbS) [8], F-actin [9], and polysaccharides [10,11]. We have already reported the polymerization of trans-4-hydroxycinnamic acid (4HCA: p-coumaric acid) as a phytomonomer, which exists in plant cell walls as an intermediate metabolite of lignin and is produced by photosynthetic bacteria [12]. Furthermore, 4HCA can also be synthesized by the enzymatic conversion of tyrosine via direct deamination [13]. The 4HCA homopolymer (P4HCA) is a main chain type of rigid homopolymer without a side chain, and showed thermotropic LC properties, whereas conventional polymers did not show thermotropic properties without the copolymerization of more than two monomers [14]. Therefore, 4HCA is expected to show strong mesogenicity to give its copolymers thermotropic properties, and thus far we investigated the copolymer characteristics such as LC behavior, LC-induced photoreactivity, and oriented cell extension on their LC fibers [14-16]. If 4HCA is copolymerized with a biomonomer containing a chromophore, then new bio-based materials showing LC-related photoemission may be prepared.
In this study, we report the synthesis and characterization of the copolymer poly{3-benzylidene amino-4-hydroxybenzoic acid (3,4-BAHBA)-co-trans-4-hydroxycinnamic acid (4HCA: trans-coumaric acid)} (Poly(3,4-BAHBA-co-4HCA)). 3,4-BAHBA is a functional biomonomer synthesized by the reaction of benzaldehyde (a biochemical) with 3-amino-4-hydroxybenzoic acid (3,4AHBA), which can be extracted from Streptomyces griseus, a microorganism suitable for the mass-production of various biomolecules [17]. 3,4-BAHBA has a Schiff-based moiety showing visible light absorption and rigid components, and 4HCA is a suitable comonomer for the production of high-performance polymers. Here, we report the relationship between the structural ordering and the photoreaction of Poly(3,4-BAHBA-co-4HCA)s of various compositions.
2. Results and Discussion
2.1. Syntheses
The monomer 3,4-BAHBA was synthesized by the reaction of 3,4-AHBA with benzaldehyde (Scheme 1). The preparation of the copolymer Poly(3,4-BAHBA-co-4HCA)s was performed according to Jin's method (Scheme 2) [18]. Table 1 shows the synthetic conditions and copolymer compositions of the Poly(3,4-BAHBA-co-4HCA)s. A mixture of 3,4-BAHBA and 4HCA was stirred at 150 ° C. The solution was yellow and transparent initially, but when the temperature increased to 180–200 °C to evaporate the acetic acid, the solution changed to a slightly brown color and began to show increased viscosity. We stopped the reaction when the reaction mixture became too viscous to stir further. The purified products were obtained as an ocher powder, which was dissolved in concentrated sulfuric acid (H2SO4) and pentafluorophenol. When the 4HCA ratio was 50 mol% or lower, the copolymers were dissolved in various solvents such as Tetrahydrofuran (THF), N,N-Dimethylformamide (DMF), Dimethyl sulfoxide (DMSO), N-methylpyrrolidone (NMP), and chloroform (Table 2). The incorporation of 3,4-BAHBA into the P4HCA increased the solubility of the copolymers.
Figure 1 shows the FT-IR spectra of the monomers and copolymers. IR peaks assigned to the double bonds (vC=C: 1,626, 1,633, and 1,635 cm−1), azomethine (vC=N: 1,625, 1,632, 1,633 cm−1), and aromatic ring (vp−φ: 1,599, 1,600, and 1,601 cm−1) appeared in the spectra for the monomers of 3,4-BAHBA and 4HCA and for the polymers of Poly(3,4-BAHBA), P4HCA, and Poly(3,4-BAHBA-co-4HCA). On the other hand, the carboxyl IR peaks observed in the spectra of the monomers of 3,4-BAHBA (vC=O: 1,664 cm−1) and 4HCA (vC=O: 1,668 cm−1) disappeared in those copolymers. Instead, the IR peaks assigned to the ester groups (vC=O: 1,733, 1,736, and 1,737 cm−1) appeared, indicating the conversion of the carboxylic acids to esters. The shoulders of Poly(3,4-BAHBA) (1,676 cm−1) and Poly(3,4-BAHBA-co-4HCA) (1,672 cm−1) would be assigned to the terminal carboxyl groups. Figure 2 shows a representative 1H-NMR spectrum of Poly(3,4-BAHBA-co-4HCA) with a 4HCA composition of 50 mol% in DMSO-d6. Multiple peaks in the region of the chemical shift of δ = 8.74 ppm (azomethine proton), δ = 8.12–8.13 ppm (aromatic protons), δ = 7.83–7.95 ppm (aromatic protons and β-CH), δ = 7.12–7.67 ppm (aromatic protons), and δ = 6.85–6.94 ppm (α-CH) were assigned. Additionally, we note that the vicinity of the attributed “c” proton in the 1H-NMR was the NH peak at the end of Poly(3,4-BAHBA-co-4HCA) chains whose molecular weights were not so high according to the results of the gel permeation chromatography (GPC) study. The compositions of 4HCA in the copolymers were calculated by the integral ratios for peak area of (a) and (b) (Table 1). 1H-NMR spectroscopy in DMSO-d6 demonstrated the incorporation of both monomers into the polymer backbone, and the 4HCA composition in copolymer can be estimated by the integration ratio of the aromatic proton signals of the individual units. The results are summarized in Table 1. The monomer composition in the feed was close to the monomeric unit composition in the copolymers, indicating the successful formation of the copolymers.
The average molecular weights of the Poly(3,4-BAHBA-co-4HCA)s were measured in THF by GPC (See Table S1 in the Supporting Information). Poly(3,4-BAHBA) and Poly(3,4-BAHBA-co-4HCA)s with in-feed 4HCA compositions of 25 and 50 mol% showed a single GPC peak, indicating Mw values of 2,300, 2,500, and 2,600, respectively (Mw/Mn = ca. 1.1). The molecular weights of the other copolymers with higher 4HCA compositions were not measured because of their low solubility in THF. However, we expected their molecular weights to be similar to those of Poly(3,4-BAHBA) and Poly(3,4-BAHBA-co-4HCA)s with in-feed 4HCA compositions of 25 and 50 mol%, because the viscosity of their concentrated H2SO4 solution was similar.
2.2. Liquid Crystalline Properties
The thermotropic properties were investigated by crossed-polarizing microscopic observation and thermogravimetric analysis/differential scanning calorimetry (TGA/DSC) measurements (See Figure S1 and Table S2 in the Supporting Information). The glass transition temperatures, Tg, of the copolymers ranged between 125 ° C and 135 ° C whereas the melting temperature, Tm, ranged between 215 ° C and 220 ° C. The 10% weight-loss temperature, T10, ranged between 300 ° C and 350 ° C. The Tg values of the copolymers were higher than the values of degradable bio-based polymers reported thus far [19]. Moreover the Poly(3,4-BAHBA-co-4HCA)s have good heat resistance properties for use in engineering thermoplastics.
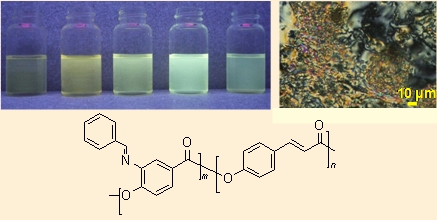
Figure 3 shows the phase diagram of Poly(3,4-BAHBA-co-4HCA)s of various compositions. These results indicated that in Poly(3,4-BAHBA-co-4HCA)s with the 4HCA compositions of 55 mol% and higher, a schlieren texture with two and four brushes could be observed (inset picture). Since the brush lines were accompanied by point defects, the LC was identified as the nematic phase. Unexpectedly, the nematic fluid also solidified at 300 ° C on average, showing a transformation of its microscopic texture from the schlieren to crystalline needles. This phenomenon was also found in our previous study on P4HCA homopolymer [14], and we thought that the crystallization was caused by a post-reaction such as acetyl group additions to double-bonds. As a whole, the phase diagram showed that the melting point of the copolymers can be controlled by a change in the composition, while retaining their liquid crystalline properties. In addition, one can see that the high percentage of 4HCA (low percentage of 3,4-BAHBA) in the composition resulted in liquid crystalline copolymers, although Poly(3,4-BAHBA) is not a liquid crystalline homopolymer.
Figure 4(a–c) shows crossed-polarizing microscopic photos of Poly(3,4-BAHBA-co-4HCA) with a 4HCA composition of 75 mol%, taken under cross-nicol polarimetry at 250 °C, and the mechanical orientation of the sample was demonstrated by shearing [20,21]. Figure 4(a) shows crossed-polarizing microscopic images taken under cross-nicol, and in Figure 4(b,c) a first-order retardation plate (λ = 530 nm) was inserted into the light path. The birefringence was negative, as evidenced by both subtractive birefringence [blue color, Figure 4(b)] in the film running from the upper left to the lower right, and by additive birefringence [orange color, Figure 4(c)] in the shearing direction from the upper right to the lower left. The negative birefringence suggests that Poly(3,4-BAHBA-co-4HCA)s with a 4HCA composition of 75 mol% show an orientation of the polymer main-chains along the shear direction. Other samples of Poly(3,4-BAHBA-co-4HCA)s with the 4HCA compositions of 60 and 100 mol% also oriented by shearing. If one thermally prepared the liquid crystal state of the copolymer and then quenched it, the LC texture was retained, indicating that the liquid crystal can be fixed on a glass without crystallization.
2.3. Photoluminescence
The photograph shown in Figure 5 demonstrated the visual photoluminescence of the Poly(3,4-BAHBA-co-4HCA)s in an NMP solution at a concentration of 0.1 g/L. Those solutions were transparent and faint yellow, but emitted various colors depending on the copolymer composition when ultraviolet (UV) light (λ = 365 nm) was irradiated. In order to quantify these colors, we recorded the photoluminescence spectra. Figure 6 shows the photoluminescence excitation spectra (left), and emission spectra (right), and Table 3 shows the photoluminescence excitation λmax (ex), and emission λmax (em) of Poly(3,4-BAHBA-co-4HCA)s of various molar ratios. From Figure 6, one can see that the Poly(3,4-BAHBA-co-4HCA) with a 4HCA composition of 75 mol% showed the highest intensity of all of the samples prepared here. The wavelength of the excitation peaks of the Poly(3,4-BAHBA-co-4HCA) with a 4HCA composition of 75 mol% ranged between those of both homopolymers, whereas the wavelength of the emission peak was almost the same as the P4HCA homopolymers. This phenomenon implies that the 3,4-BAHBA units play the role of an antenna for light energy with a wide wavelength range, and transmit this energy into the 4HCA units to emit strongly. As a consequence, the Poly(3,4-BAHBA-co-4HCA)s have photoluminescence properties.
The fluorescence spectra of orientation films of Poly(3,4-BAHBA-co-4HCA)s with the 4HCA compositions of 75 and 100 mol% were measured while changing the directions of the polarizer and analyzer [Figure 7(a,b)]. When the polarizer and analyzer directions are both parallel to the film orientation, the emission intensity is the highest. Otherwise, if one of the polarizers or analyzers was perpendicular to the sample orientation, then the intensities were very low. It is noteworthy that even if the polarizer and analyzer direction was parallel, the intensity was low in the film orientation direction perpendicular to either unit. This result supports simple reflection based on a mirror effect of the sample would be insufficient. The emission intensities at 469 nm in the copolymers with the 4HCA compositions of 75 and 100 mol% were 23 and 30, respectively. From these intensity data, we normalized the emission intensity per 4HCA unit to 0.31 and 0.30, suggesting that the 3,4-BAHBA units slightly enhanced the intensity of polarized light from the 4HCA units, even a shortened 4HCA continuous segment.
3. Experimental Section
3.1. Materials
3,4-AHBA (Kanto chemical, Co. Inc.) and 4HCA (Kitamura Chemicals, Co. Ltd.) monomers were used as received. Acetic anhydride (Kanto chemical, Co. Inc.) was used as an acetylation agent and disodium hydrogen phosphate (Kanto chemical, Co. Inc.) as a polymerization catalyst were used as received. DMSO-d6 (Sigma-Aldrich Co.) was used as an NMR solvent, also as received.
3.2. Synthesis of 3,4-BAHBA
A mixture of 3,4-AHBA (3.0 g) and benzaldehyde (30 mL) was heated at 150 °C to create a homogeneous liquid, which was stirred for 1 hour under nitrogen in an oil bath. After this reaction, the mixture was cooled to room temperature, and the yellow crystals were filtered, washed with ethanol, and vacuum-dried overnight at room temperature. Yield: 2.6 g (55%), mp: 237 °C. IR (in cm−1): 1,664 (ν C=O), 1,625 (νc=n) (See Figure 1). 1H-NMR (400 MHz, DMSO-d6, δ, ppm, TMS): 12.59 (s, 1H, COOH, He), 9.88 (s, 1H, OH, Hd), 8.75 (s, 1H, CH=N, Hf), 8.06 (dd, J = 7.4, 1.6 Hz, 2H, aryl, Hg), 7.73 (d, J = 2.0 Hz, 1H, aryl, Ha), 7.70 (dd, J = 8.4, 2.0 Hz, 1H, aryl, Hc), 7.57-7.50 (m, 3H, aryl, Hh and Hi), 6.97 (d, J = 8.0 Hz, 1H, aryl, Hb) (See Figure S2 in the Supporting Information). 13C-NMR (100 MHz, DMSO-d6, δ, ppm, TMS): 167.3 (COOH, Ca), 160.7 (CN, Ch), 155.3 (aryl, Ce), 138.1 (aryl, Cd), 136.2 (aryl, Ci), 131.6 (aryl, Cl), 129.1 (aryl, Cj), 129.1 (aryl, Cg), 128.7 (aryl, Ck), 122.1 (aryl, Cb), 120.6 (aryl, Cc), 116.0 (aryl, Cf) (See Figure S3 in the Supporting Information). ESI FT-ICR MS [M+H]+: 242.08133, Calcd. for C14H12NO3: 242.08117. [M+Na]+: 264.06324, Calcd. for C14H11NO3Na: 264.06311.
3.3. Syntheses of Poly(3,4-BAHBA-co-4HCA)s
The copolymer with a 4HCA in-feed composition of 50 mol% was prepared as follows. Monomers of 3,4-BAHBA (2.1 mmol) and 4HCA (2.1 mmol) were stirred at 150 °C for 3 hours in the presence of 5 mL acetic anhydride as a condensation reagent and disodium hydrogen phosphate as a catalyst for the transesterfication. After 3 hours, the reactants were stirred at an elevated temperature of 180–200 °C for 30 min to distill acetic acid, and then vacuumed at 200 °C for 15 min until the reaction mixture became too viscous to stir further. After this reaction, the product was dissolved in DMF and purified by reprecipitation over methanol, filtered, washed with methanol and vacuum-dried overnight at 80 °C. The yield was 59%. The copolymers with other 4HCA compositions were prepared by an analogous procedure.
Number- and weight-average molecular weights (Mn and Mw) were determined by gel permeation chromatography at 40 °C (GPC JASCO GULLIVER SERIES UV-970; column, Shodex GPC KF-801 and KF-802; eluant, THF), and calibrated with polystyrene standards at a flow rate of 1.0 mL/min. The results are summarized in Table S1 (See Table S1 in the Supporting Information).
3.4. Measurements
Fourier transform infrared spectra (FT-IR) of 3,4-BAHBA, 4HCA, and its polymer were recorded on a Perkin-Elmer Spectrum One spectrometer using a diamond-attenuated total reflection (ATR) accessory. 1H and 13C NMR spectra were measured in a DMSO-d6 solution by an NMR spectrometer (Bruker, Avance III) at 400 MHz. 1H and 13C NMR spectra chemical shifts in parts per million (ppm) were recorded downfield from 2.5 ppm and 39.5 ppm using DMSO as an internal reference.
Fourier transform ion cyclotron resonance mass spectra (FT-ICR MS) with an electrospray ionization system (ESI) were recorded on a Solari X, Bruker Daltonics Inc. A methanol solution of 3,4-BAHBA was prepared as a specimen by a 50-fold dilution of a saturated solution.
The phase transition of Poly(3,4-BAHBA-co-4HCA)s was observed by crossed-polarizing microscopy. The samples were sandwiched between two glass plates, and heated at a rate of 10 °C/min by a METTLER TOLEDO FP82HT Hot Stage (Greifensee, Switzerland). The melting temperature (Tm) and the liquid crystalline phase were observed directly by microscopy.
The thermotropic behavior was confirmed by differential scanning calorimetry (DSC) measurements (EXSTAE6100; Seiko Instruments Inc., Chiba, Japan) at a scanning ratio of 10 °C /min from 50 to 250 °C in a nitrogen atmosphere. The Tm of the new compound 3,4-BAHBA was measured by DSC. The Tg and Tm of the copolymers were obtained from the DSC curves of the second heating cycle. Thermal degradation was also analyzed by thermogravimetric analysis (TGA; SSC/5200 SII Seiko Instruments Inc.) by heating from 50 to 750 °C at a rate of 10 °C /min under a nitrogen atmosphere.
Photoluminescence excitation and emission spectra of the polymer solutions were obtained with a spectrophotometer. The polymer concentration in NMP was 0.1 g/L. Poly(3,4-BAHBA-co-4HCA)s solutions with 4HCA compositions of 75 and 100 mol% were filtered because they were partially soluble in NMP.
3.5. Photoluminescence of Oriented Film
Figure 8 illustrates the optical train used for the photoluminescence dichroism measurements, which was performed using a fluorophotometer (FP-6500; Jasco Instruments Inc., Japan). The samples were mounted on a glass slide, and were heated by a Hot Stage. Just above the Tm, glass plates were used to sandwich the sample on the arrowed direction, and they were super-cooled to room temperature to create an oriented film coating the glass. We then inserted the film between the polarizer and the analyzer.
4. Conclusions
In order to prepare new functional bio-based polymers with LC properties, poly{3-benzylidene amino-4-hydroxybenzoic acid (3,4-BAHBA)-co-trans-4-hydroxycinnamic acid (4HCA: trans-coumaric acid)} (Poly(3,4-BAHBA-co-4HCA)) was synthesized by a thermal polycondensation of 4HCA and 3,4-BAHBA, which was synthesized by a reaction of 3-amino-4-hydroxybenzoic acid (3,4-AHBA) with benzaldehyde. When the 4HCA compositions of Poly(3,4-BAHBA-co-4HCA)s were above 55 mol%, the copolymers showed a nematic liquid crystalline phase due to the mesogenic effects of the continuous 4HCA units. Furthermore, the copolymers in an NMP solution showed photoluminescence under 365 nm, and the corresponding oriented films of Poly(3,4-BAHBA-co-4HCA)s with a 4HCA composition of 75 mol% emitted polarized light, as confirmed by fluorescent spectroscopy equipped with paralleled and crossed polarizers. Thus, we prepared new, rigid-rod oligomers with LC-related photoemission properties derived from aromatic structures in biomolecules available from microorganisms.
Supplementary Material
polymers-03-00861-s001.pdf









| 3,4-BAHBA/4HCAa (mol%) | 3,4-BAHBA (mmol) | 4HCA (mmol) | 3,4-BAHBA/4HCAb (mol%) | Yield (%) |
|---|---|---|---|---|
| 100/0 | 2.1 | 0 | 100/0 | 37 |
| 75/25 | 2.1 | 0.69 | 66/34 | 55 |
| 50/50 | 2.1 | 2.1 | 53/47 | 59 |
| 25/75 | 2.1 | 6.2 | 38/62 | 73 |
| 0/100 | 0 | 3.0 | 0/100 | 85 |
a The numbers refer to the molar percentage of in-feed compositionb Molar ratios were estimated by 1H-NMR spectra.
| Solvents | 3,4-BAHBA | 4HCA | Poly(3,4-BAHBA-co-4HCA) | ||||
|---|---|---|---|---|---|---|---|
| 3,4-BAHBA/4HCA (mol%) | |||||||
| 100/0 | 75/25 | 50/50 | 25/75 | 0/100 | |||
| Methanol | + | ++ | − | − | − | − | − |
| Ethanol | + | ++ | − | − | − | − | − |
| Acetone | + | ++ | +− | +− | +− | +− | +− |
| THF | ++ | ++ | ++ | ++ | ++ | +− | +− |
| DMF | ++ | ++ | ++ | ++ | ++ | +− | +− |
| DMAc | ++ | ++ | ++ | ++ | ++ | +− | +− |
| NMP | ++ | ++ | ++ | ++ | ++ | +− | +− |
| DMSO | ++ | ++ | ++ | ++ | ++ | +− | +− |
| TFA | ++ | + | ++ | ++ | ++ | +− | +− |
| H2SO4 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Pentafluorophenol | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| H2O | − | − | − | − | − | − | − |
| Chloroform | − | − | ++ | ++ | ++ | − | − |
| Toluene | − | − | − | − | − | − | − |
| Hexane | − | − | − | − | − | − | − |
++: soluble at r.t., +−: partly soluble, −: insoluble, +: soluble only by heating. THF: Tetrahydrofuran, DMF: N,N-Dimethylformamide, DMAc: N,N-Dimethylacetamide, NMP: N-methylpyrrolidone, DMSO: Dimethyl sulfoxide, TFA: Trifluoroacetic acid.
| 3,4-BAHBA/4HCA | λmax (ex) (nm) | λmax (em) (nm) |
|---|---|---|
| 100/0 | 402 | 484 |
| 75/25 | 388 | 487 |
| 50/50 | 378 | 468 |
| 25/75 | 374 | 466 |
| 0/100 | 362 | 459 |
Acknowledgments
This research was financially supported from a Grant-in-Aid for Comprehensive Support Programs for Creation of Regional Innovation Science and Technology Incubation Program in Advanced Regions “Practical Application Research” (Kaneko project).
References
- Dollings, P.J.; Hird, M. Introduction to Liquid Crystals; Tayor & Francis: London, UK, 1997. [Google Scholar]
- Chandroasekhar, S. Liquid Crystals, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Morgan, P.W. Synthesis and properties of aromatic and extended chain polyamides. Macromolecules 1977, 10, 1381–1390. [Google Scholar]
- Choe, E.W.; Kim, S.N. Synthesis, spinning, and fiber mechanical properties of poly(p-phenylenebenzobisoxazole). Macromolecules 1981, 14, 920–924. [Google Scholar]
- Robinson, C. Liquid-crystalline structures in polypeptide solutions. Tetrahedron 1961, 13, 219–234. [Google Scholar]
- Livolant, F. Lines in liquid crystalline phases of biopolymers. J. Phys. Fr. 1989, 50, 1729–1741. [Google Scholar]
- Spencer, M.; Fuller, W.; Wilkins, M.H.F.; Brown, G.L. Determination of the helical configuration of ribonucleic acid molecules by X-Ray diffraction study of crystalline amino-acid-transfer ribonucleic acid. Nature 1962, 194, 1014–1020. [Google Scholar]
- Perutz, M.F.; Liquori, A.M.; Eirich, F. X-Ray and solubility studies of the hæmoglobin of sickle-cell anæmia patients. Nature 1951, 167, 929–931. [Google Scholar]
- Coppin, C.M.; Leavis, P.C. Quantitation of liquid-crystalline ordering in F-actin solutions. Biophys. J. 1992, 63, 794–807. [Google Scholar]
- Giraud-Guille, M.M. Twisted liquid crystalline supramolecular arrangements in morphogenesis. Int. Rev. Cytol. 1996, 166, 59–101. [Google Scholar]
- Suto, S.; Umeda, T. Swelling behavior of crosslinked hydroxypropylcellulose films retaining cholesteric liquid crystalline order, II. Effects of temperature, solvent, and pH. Angew. Makromol. Chem. 1999, 264, 60–64. [Google Scholar]
- Hoff, W.D.; Dux, P.; Hard, K.; Devreese, B.; Nugteren-Roodzant, I.M.; Crielaard, W.; Boelens, R.; Kaptein, R.; van Beeumen, J.; Hellingwerf, K.J. Thiol ester-linked p-coumaric acid as a new photoactive prosthetic group in a protein with rhodopsin-like photochemistry. Biochemistry 1994, 33, 13959–13962. [Google Scholar]
- Wightman, F.; Chisholm, M.D.; Neish, A.C. Biosynthesis of tryptophan and gramine in young barley shoots. Photochemistry 1961, 1, 30–37. [Google Scholar]
- Kaneko, T.; Matsusaki, M.; Hang, T.T.; Akashi, M. Thermotropic liquid-crystalline polymer derived from natural cinnamoyl biomonomers. Macromol. Rapid Commun. 2004, 25, 673–677. [Google Scholar]
- Kaneko, T.; Hang, T.T.; Matsusaki, M.; Akashi, M. Biodegradable LC oligomers with cranked branching points form highly oriented fibrous scaffold for cytoskeletal orientation. Chem. Mater. 2006, 18, 6220–6226. [Google Scholar]
- Kaneko, T.; Hang, T.T.; Shi, D.J.; Akashi, M. Environmentally degradable, high-performance thermoplastics from phenolic phytomonomers. Nat. Mater. 2006, 5, 966–970. [Google Scholar]
- Suzuki, H.; Ohnishi, Y.; Furusho, Y.; Sakuda, S.; Horinouchi, S. Novel benzene ring biosynthesis from C3 and C4 primary metabolites by two enzymes. J. Biol. Chem. 2006, 281, 36944–36951. [Google Scholar]
- Jin, X.; Carfagna, C.; Nicolais, L.; Lanzetta, R. Synthesis, characterization, and in vitro degradation of a novel thermotropic ternary copolyester based on p-Hydroxybenzoic acid, glycolic acid, and p-Hydroxycinnamic acid. Macromolecules 1995, 28, 4785–4794. [Google Scholar]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar]
- Yamaoka, K.; Kaneko, T.; Gong, J.P.; Osada, Y. Liquid crystalline gels. 3. Role of hydrogen bonding in the formation and stabilization of mesophase structures. Macromolecules 2001, 34, 1470–1476. [Google Scholar]
- Kaneko, T.; Yamaoka, K.; Osada, Y.; Gong, J.P. Thermoresponsive shrinkage triggered by mesophase transition in liquid crystalline physical hydrogels. Macromolecules 2004, 37, 5385–5388. [Google Scholar]
- Electronic Supplementary Information (ESI) Available TGA and DSC thermogram of Poly(3,4-BAHBA), monomer composition, molecular weight, and thermal analysis data of Poly(3,4-BAHBA-co-4HCA)s, 1H-NMR and 13C-NMR spectra of 3,4-BAHBA.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kan, K.; Kaneko, D.; Kaneko, T. Polarized Emission of Wholly Aromatic Bio-Based Copolyesters of a Liquid Crystalline Nature. Polymers 2011, 3, 861-874. https://doi.org/10.3390/polym3020861
Kan K, Kaneko D, Kaneko T. Polarized Emission of Wholly Aromatic Bio-Based Copolyesters of a Liquid Crystalline Nature. Polymers. 2011; 3(2):861-874. https://doi.org/10.3390/polym3020861
Chicago/Turabian StyleKan, Kai, Daisaku Kaneko, and Tatsuo Kaneko. 2011. "Polarized Emission of Wholly Aromatic Bio-Based Copolyesters of a Liquid Crystalline Nature" Polymers 3, no. 2: 861-874. https://doi.org/10.3390/polym3020861
APA StyleKan, K., Kaneko, D., & Kaneko, T. (2011). Polarized Emission of Wholly Aromatic Bio-Based Copolyesters of a Liquid Crystalline Nature. Polymers, 3(2), 861-874. https://doi.org/10.3390/polym3020861