Incorporation of Hyperbranched Supramolecules into Nafion Ionic Domains via Impregnation and In-Situ Photopolymerization
Abstract
: Nafion membranes were impregnated with photocurable supramolecules, viz., hyperbranched polyester having pendant functional carboxylic acid groups (HBPEAc-COOH) by swelling in methanol and subsequently photocured in-situ after drying. Structure-property relationships of the HBPEAc-COOH impregnated Nafion membranes were analyzed on the basis of Fourier transform infrared (FTIR) spectroscopy, solid-state nuclear magnetic resonance (SSNMR) and dynamic mechanical analysis (DMA). FTIR and SSNMR investigations revealed that about 7 wt % of HBPEAc-COOH was actually incorporated into the ionic domains of Nafion. The FTIR study suggests possible complexation via inter-species hydrogen bonding between the carboxylic groups of HBPEAc-COOH and the sulfonate groups of Nafion. The α-relaxation peak corresponding to the glass transition temperature of the ionic domains of the neat Nafion-acid form was found to increase from ~100 to ~130 °C upon impregnation with enhanced modulus afforded by the cured polyester network within the ionic domains. The AC impedance fuel cell measurement of the impregnated membrane exhibited an increasing trend of proton conductivity with increasing temperature, which eventually surpassed that of neat Nafion above 100 °C. Of particular importance is that the present paper is the first to successfully incorporate polymer molecules/networks into the Nafion ionic domains by means of impregnation with hyperbranched supramolecules followed by in-situ photopolymerization.1. Introduction
With the emergence of proton fuel cells for clean energy, perfluorinated ionomer membrane, commercially known as Nafion, has received widespread attention from researchers in both academia and industry. By virtue of its outstanding proton conductivity [1,2], Nafion is currently regarded as the benchmarked polymer electrolyte membrane (PEM). One major drawback is that its operation temperature is limited to 70–80 °C, above which the proton conduction progressively gets worse. In the actual proton fuel cell operation, a numbers of PEM fuel cells have to be staked to obtain desirable power output [3]. An evitable consequence is that the cell temperature rises significantly beyond the optimum operating temperature of Nafion. Hence, there is a growing interest for developing new PEM materials capable of functioning at high temperatures under the actual proton fuel cell operations.
There are several attempts to remedy the aforementioned shortcomings of Nafion perfluorinated ionomer membranes, especially to increase proton conduction at elevated temperatures [4,5]. One notable approach is the in-situ impregnation (or doping) by means of incorporating functional small molecules such as ionic liquids within the Nafion ionic domains/clusters [6-9]. Infusion of ionic liquids into Nafion has afforded considerable improvement in ionic conduction. In addition, the occurrence of specific interaction has been identified, i.e., hydrogen and/or ionic bonding between these small molecules and the sulfonate groups of Nafion has helped retaining these functional small molecules during use [8,9].
The main idea of the present research is to incorporate functional photopolymerizable supramolecules into the ionic domains and subsequently polymerize it in-situ to form functional polymer networks in order to prevent any potential leaching in actual fuel cell operations. Another goal is to improve proton conduction at elevated temperatures where Nafion performance has declined considerably.
In this manuscript, we have incorporated solid supramolecules such as photocurable hyperbranched (HB) polyester terminated with functional carboxylic acid groups, namely HBPEAc-COOH [10], into the micro-phase separated ionic domains of Nafion via swelling in methanol. It can be anticipated that the terminal carboxylic acid of HB will not only enhance ion exchange capacity, but also impart inter-species hydrogen bonding with the sulfonate groups of Nafion that provides paths for proton conduction. Subsequently, photo-crosslinking was performed in-situ to retain the modifier supramolecules within the ionic domains and also to enhance its proton conduction above the current operating temperature of Nafion in the reduced water environment. Suppression of the excessive swelling upon hydration is also expected through occupying available space within the ionic domains of Nafion by these supramolecules. The incorporated amount of HB supramolecules was evaluated by means of Fourier transform infrared (FTIR). The physical and structural characterization of the HB impregnated Nafion was performed using solid-state nuclear magnetic resonance (SSNMR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), dynamic mechanical analysis (DMA), and AC impedance measurements under the proton fuel cell environment. Of particular importance is that the present work will be the first to successfully incorporate large polymer molecules into the ionic domains of Nafion through impregnation and subsequent in-situ polymerization of photopolymerizable hyperbranched polyester [10]. More importantly, the present approach will afford better dimensional and thermal stability of the impregnated membrane with improved proton conduction during high temperature proton fuel cell operations relative to the unmodified Nafion.
2. Results and Discussion
Prior to incorporation into the Nafion membranes, the physical and chemical characterization of HB polyester was conducted by means of DSC, FTIR and SSNMR. Figure 1 shows the DSC thermograms of pure HB polyesters before and after photocrosslinking. The movement of the glass transition (Tg), i.e., the mid-point of the transition of pure HB from 79 to 95 °C after photocuring indicates that the overall motion of the HB polyester chains becomes restricted. The act of photocuring leads to the formation of a chemically crosslinked network structure with restricted chain motions.
The overlay FTIR spectra of neat HBPEAc-COOH, HBPEAc-COOH with the initiator before photocuring and HBPEAc-COOH after photocuring are shown in Figure 2. Note that the amount of Irgacure 907 added was 3 wt % relative to the weight of HBPEAc-COOH. It is evident that with the addition of photoinitiator to the neat HB polyester, there is no discernible change in the characteristic FTIR peaks such as 1,727 cm−1 (C=O carbonyl stretching) and/or 1,508 cm−1 (C=C stretching of the aromatic rings). A similar peak invariance can also be noticed in other characteristics bands at 1,230 cm−1 and 1,190 cm−1 corresponding to the C–O–C stretching of cyclic ether linkages of HBPEAc-COOH, and 830 cm−1 and 740 cm−1 peaks attributable to C–H bending and C=C aromatic out-of-plane bending vibrations. However, the addition of 3 wt % of photoinitiator is sufficient to achieve a high conversion (i.e., ~60%) in 4 min as demonstrated in an earlier paper [10]. Upon photocuring, the C=C stretching band of acrylate near 1,630 cm−1 decreases as it transformed to a C–C single bond after being attacked by free radicals of the photoinitiator. It should be emphasized that the HB polyester film is brittle without photocuring.
The 13C CP/MAS NMR spectra acquired from cured HBPEAc-COOH and impregnated membranes are shown in Figure 3. It should be emphasized that the peaks in these spectra arise solely from the perprotio species of HBPEAc-COOH and photoinitiator. The spectra are complex as a result of the presence of many different types of carbons in the HB polyester. The most significant differences between the spectra from neat HBPEAc-COOH and impregnated membranes are the additional peaks at 26, 52, 68 and 74 ppm that arise from the substituted morpholino portion of the photoinitiator used to crosslink the HB supramolecule.
In order to quantify the amount of hyperbranched polyester incorporated, the total signal intensity of the 13C CP/MAS NMR spectra of HBPEAc-COOH was evaluated. Correcting for the total number of transients collected and sample size, the amount of HBPEAc-COOH present in the impregnated membrane was determined to be 7 ± 1 wt %. As will be discussed later, this mass of HB polymer in the Nafion film matches the results obtained in the FTIR studies (vide infra).
The fluoropolymer backbone of the Nafion membrane was investigated to determine if there were any changes in this environment upon impregnating Nafion with HBPEAc-COOH. The 13C NMR spectrum was collected using 19F-13C CP and 19F decoupling so that the peaks in the 13C NMR spectrum unambiguously arise from the fluorocarbon component of Nafion. The 13C spectrum from Nafion, Figure 4, is consistent with those reported previously [12-14] with the peaks at 114 ppm from backbone –CF2 groups, 120 ppm from the –OCF2 group, 122 ppm from the –CF3, 112 ppm from the backbone –CF groups and 106 ppm from the –CF side chain groups. There are no noticeable changes in the chemical shifts in the 13C NMR spectra observed for the neat Nafion and impregnated membrane. These results suggest that there is little or no reactivity between the Nafion fluorocarbon backbone and HBPEAc-COOH. However, there is a slight change in linewidths, with the impregnated membrane having narrower lines than those of the neat Nafion. This minor change presumably occurs due to changes in the environment of the fluoro component as this portion of the membrane becoming more mobile. As will be discussed later, the SSNMR observations conform to the mechanical β-relaxation temperature observation which is attributable to the glass transition temperature of fluorocarbon backbone of pure Nafion. It appears that the inclusion of HB polyester in the Nafion ionic domains not only alters the environment of the ionic region, but also exerts some influence on the motion of the fluorocarbon backbone chain and/or side groups.
Nafion membranes were characterized prior to and after the infusion of the HB polyester by determining water content, HB polyester uptake levels, and the interactions between the Nafion and HB polyester. The 1H NMR spectra of neat Nafion (Figure 5) shows a single peak at 8.6 ppm, and this chemical shift corresponds to approximately 2 water molecules per SO3H group present in the Nafion membrane [15,16]. The narrow linewidth of the peak indicates that the H2O molecules are mobile. The two broad peaks in the 1H NMR spectrum from neat hyperbranched polyester, without the presence of initiator, are assigned to the aromatic protons, i.e., the downfield peak and the aliphatic protons, i.e., the upfield peak (Figure 5). After addition of the HB polymer to the Nafion membrane, the 1H NMR spectra are similar, showing two broad peaks arising from the HB polyester. The presence of the additional peak in the aliphatic region of the spectrum of the impregnated membrane is attributed to the photoinitiator. The absence of any narrow peaks in the 1H NMR spectrum from the impregnated membrane implies that there are no mobile water molecules present. This suggests that the infused HB supramolecules into the ionic regions of the Nafion presumably have replaced, if not all, some water molecules and also restricted the mobility of any remaining water molecules.
ATR-FTIR experiments were conducted in order to probe the interactions between HBPEAc-COOH and the ionic domains of Nafion, as well as to determine the estimated amounts of HBPEAc-COOH incorporated into Nafion. The FTIR spectra of neat Nafion, neat HBPEAc-COOH and impregnated membranes with different HB polyester feed ratios are depicted in Figure 6. These spectra were collected under the minimal moisture absorption at 100 °C, e.g., bound water. At a higher FTIR wavenumber range around 3,300–3,600 cm−1, the IR absorption band is extremely broad so much so that the peak position is not identifiable. This broad band implies the presence of bound water within pure Nafion. On the other hand, the cured HBPEAc-COOH exhibits a pronounced O–H stretching band (at ~3,500 cm−1) albeit broad, arising from its pendant carboxylic acid functionalities [17] which may be interacting with some bound water molecules. With impregnation of HB supramolecules into Nafion, the broad O–H stretching band remains virtually stationary with increasing HB contents from 2–10 wt % [see Figure 6(a)].
Figure 6(b) exhibits the FTIR spectrum of neat Nafion membrane showing two strong bands at 1,200 cm−1 and 1,140 cm−1, attributable to the C–F asymmetric and symmetric stretching vibrations of the main chain, respectively, along with the C–F stretching vibrations from the side chains of Nafion at 980 cm−1. As expected, there is no movement of these peaks upon incorporation of the HB polyester. Similarly, the C–F bending mode at 967 cm−1 and –CF2 rocking mode at 625 cm−1 remained stationary upon impregnation of the HB polyester in Nafion. Together with the SSNMR results, it is reasonable to infer that in the dried state there is little or no influence on the reactivity on the fluoropolymer components of the Nafion backbone upon inclusion of the HB polyester into the ionic domains.
The FTIR spectrum from neat HBPEAc-COOH exhibits a number of characteristic bands; specifically the peak at 1,727 cm−1 arising from the carbonyl stretching, whereas the peak at 1,508 cm−1 associated with the C=C stretching of the aromatic rings. The bands at 1,230 cm−1 and 1,190 cm−1 correspond to the C–O–C stretching of cyclic ether linkages of HBPEAc-COOH. The 830 cm−1 and 740 cm−1 peaks are associated with the C–H bending and C=C aromatic out-of-plane bending vibrations, respectively. The C=O band of the neat HB, which is located at 1,727 cm−1, shows a minor shift upon impregnation for about 2 ~ 3 cm−1 which is below the spectral resolution of 4 cm−1 and thus it is inconclusive.
In the case of neat Nafion, the S–O band, located at 1,057 cm−1, is slightly lower than that of th dried Nafion (1,061 cm−1) [18] which implies that some bound water might be present in the Nafio at 100 °C. According to Falk, this symmetric S–O stretching band shifts to a lower wavenumber upo hydration engaging in the water-water and/or water-ion interactions [19]. However, the S–O band neat Nafion is noticeably shifted from 1,057 cm−1 to 1,048 cm−1 in the impregnated Nafion. Th shifting of the S–O band to a lower wavenumber upon HB impregnation suggests the occurrence complexation of S–O possibly with O–H of carboxylic acid group of the HB supramolecules bridgin through some bound water molecules. A natural question is why the C=O band shows little or n movement. It may be hypothesized that when the bound water in HB interacts with S–O of Nafion, som C=O bonds may be freed up, thereby contributing to a spectral red shift. This may be compensated b the blue shift of C=O if inter-species complexation were to occur between the carboxylic groups HBPEAc-COOH and the sulfonate groups of Nafion, e.g., inter-species hydrogen bonding.
In order to quantify the estimated amount of HBPEAc-COOH incorporated in the Nafion membran two different FTIR approaches were employed. Note that the FTIR spectra were collected after wipin off any residual HBPEAc-COOH that might be deposited on the surface of Nafion membranes. Aft drying, the impregnated membrane was photocured in accordance with the procedure described in th experimental section. The first approach focuses on the peak (position) shift of S–O band with H polyester content and the second approach utilizes the integrated area under the C=O and C= aromatic peak at 1,727 cm−1 and 1,508 cm−1 to determine the amount of HBPEAc-COOH in Nafion.
As shown in Figure 7(a), the S–O band at 1,057 cm−1 moves to a lower wavenumber (i.e., about 9 cm−1) with increasing loading level of HBPEAc-COOH in Nafion. This moving trend levels off at about 5–10 wt % of HB polyester in the feed suggesting that the impregnation of HBPEAc-COOH into the Nafion ionic domain has reached a saturation level. In the second method, the area under the curves of C=O and C=C aromatic band were calculated as described before. The dashed line indicates an ideal situation, where the amount of HBPEAc-COOH in the impregnated membrane conforms exactly to that of the feed. As seen in Figure 7(b), the estimated amount of HBPEAc-COOH that is incorporated into the Nafion conforms relatively well with the ideal situation up to 5 wt % and then it saturates out after 10 wt %. Hence, the estimated amount of incorporated HBPEAc-COOH was estimated to be about 7 wt %. Based on the FTIR investigation, it may be concluded that the amount of HB polyester incorporated is about 5 to 7 wt % which is in good accord with the relative intensity quantification by SSNMR. The FTIR and SSNMR studies revealed that the Nafion membrane was indeed impregnated with the HB polyester network, through occupying the hydrophilic packets of the membrane and interacting with the SO3H groups.
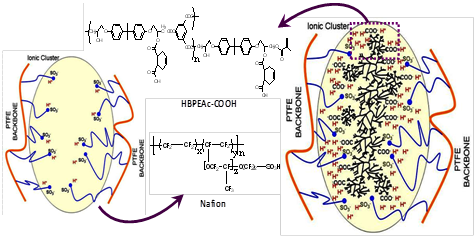
Based on the observed SSNMR and FTIR findings, a schematic drawing in Figure 8 is presented to hypothesize a plausible mechanism of aggregated structure within the ionic domains of the HB impregnated Nafion. It seems that the specific interactions such as inter-species hydrogen bonding between the –COOH of HBPEAc-COOH and the –SO3H of Nafion is prevalent. Moreover, the HB polyester networks appear isolated from the surrounding fluorocarbon matrix, having virtually little or no direct interaction with the fluorocarbons except for contributing to a slight gain in mobility of the backbone chains. The impregnated HB supramolecules will take away some of the space within the ionic domains and thus limited space will be available for water molecules to occupy.
Figure 9(a) shows the plot of water uptake as a function of soaking time for both neat and impregnated membranes at room temperature. The water uptake of neat Nafion in acid form is crucial [20] and was found to absorb up to 37 wt %. Elliot et al. [21] demonstrated the swelling of the ionic domains by water in turn plasticizes the fluorocarbon matrix. As shown in Figure 9(a), this swelling can be suppressed considerably by incorporating HBPEAc-COOH into the Nafion, i.e., the water uptake drops from 15 wt % to 10 wt % as the composition of HBPEAc-COOH in feed increases from 3 wt % to 10 wt %. Note that the above water uptake experiment is important to evaluate the dimensional stability characteristics, since the physical dimensions of both impregnated membrane and neat Nafion are difficult to compare due to the severe warping displayed especially by the pure Nafion.
In Figure 9(b) is shown the number of moles of water per mole of sulfonic acid site (λ) as a function of HBPEAc-COOH composition in feed. In neat Nafion-acid, up to 22 moles of water are absorbed per mole of sulfonic acid site after 24 h of soaking; this number increases with water temperature [22]. However, in the case of the impregnated membrane, only seven moles of water can be absorbed by one sulfonic acid site as the number of moles of water saturates out at ~10 wt % of HBPEAc-COOH in feed. According to Paddison et al. [23], the ab initio calculation for the elucidation of the proton transport mechanism of Nafion revealed that only 6 H2O/SO3− are needed to exhibit the complete proton dissociation resulting in efficient proton conduction. Upon hydration, the fluorocarbon matrix undergoes reorganization, which imposes large internal stresses on the membrane resulting in warping as depicted in the inset right picture of Figure 9(b). Consequently lower swelling capability and minimized internal stress within the impregnated membrane leads to better dimensional stability upon hydration as seen in the inset left picture of Figure 9(b). It can be envisioned that the sulfonic acid sites have been surrounded with HBPEAc-COOH molecules and this shielding of the ionic sites lowers the hydrophilicity of the membrane. The formation of the cured HB polyester network has contributed to improved dimensional stability of the Nafion membrane.
Furthermore, Figure 9(b) demonstrates that the ion exchange capacity (IEC) of impregnated membranes exceeds the IEC of neat Nafion, i.e., ~0.92 meq g−1, showing increasing trend of the IEC value with addition of HB. The IEC for the impregnated membrane, however, saturated out at 5 wt % of HB in feed with a maximum value of ~1.41 meq g−1. The enhancement of IEC may be attributed to the increase in the number of acidic protons, i.e., the carboxylic acid sites in HB within the ionic clusters.
Next, DMA was performed to mimic the viscoelastic properties of the impregnated membrane by analyzing the relaxation temperature of the ionic domain, i.e., α-relaxation. Figure 9 shows the plots of storage modulus, E′ and tan δ curves versus temperature for 10% HBPEAc-COOH impregnated Nafion and neat Nafion membranes. In the neat Nafion-acid, there are three relaxations termed γ, β and α-relaxations in ascending order of temperature from −130 to 150 °C that were observed at −70, 15 and 100 °C respectively. According to Kyu et al. [24], these γ-, β- and α-relaxations can be assigned to the –CF2 local motions, glass transition of Nafion matrix and glass transition of the ionic domain, respectively. Underwater stress relaxation of neat Nafion in acid and sodium forms showed that the α-relaxation temperature is profoundly affected by the presence of water in the ionic regions [25]. One drawback of the neat Nafion-acid form is that the hydrophilic ionic domains become extremely mobile above this α-relaxation temperature and thus the interconnected channels may eventually be disrupted upon prolonged exposure to elevated temperature.
The impregnation of Nafion by HBPEAc-COOH raised the α-relaxation temperature from 100 to 130 °C, thereby improving the thermal stability of the membrane. This improved α-relaxation temperature may be due to the inter-species hydrogen bonding interaction and/or the chemical crosslinking of the supramolecules contributing to the enhanced thermal stability as demonstrated earlier in the FTIR study. In the inset of Figure 10, are shown the pictures of membrane samples taken after DMA experiments at 150 °C. It is striking to witness the brownish color of neat Nafion, i.e., the manifestation of thermal instability of protonated ionic domains, turned into light beige (i.e., inherent Nafion color) in the impregnated membrane, implying improved thermal stability at 150 °C.
With descending temperature, an additional relaxation peak can be discerned at ~90 °C, Figure 10. This peak temperature is analogous to the DSC Tg of cured neat HB polyester and thus it is reasonable to assign it to the glass transition of cured HBPEAc-COOH within the impregnated membrane. The original β-relaxation peak of neat Nafion, i.e., the glass transition of the fluorocarbon backbone of Nafion, slightly decreases from 15 to 5 °C (Figure 10), but the tan δ value becomes more pronounced. However, it should be pointed out that this β-relaxation peak is considerably overlapped with the onset of the Tg peak of HB supramolecules. This overlap might have contributed to the enhanced movement of the fluorocarbon backbone chains, thereby increasing intensity of the β-relaxation peak. Recall the finding of the SSNMR of 19F-13C CP/MAS in Figure 4 that shows virtually no reactivity between the HB supramolecules and the fluorocarbon backbone matrix in the dried state, except the narrowing of the linewidth of the –CF2 backbone of Nafion. This SSNMR narrowing indicates that the fluorocarbon chain is becoming more mobile, which is consistent with the enhanced strength of the mechanical β-relaxation peak caused by the HB impregnation (Figure 10).
Another important observation is the increase in the storage modulus values of the impregnated membrane. As can be seen in Figure 10, the modulus of the HBPEAc-COOH impregnated Nafion membrane increases over one order of magnitude at lower temperatures, which may be attributed to the HBPEAc-COOH network. Although both storage moduli of impregnated Nafion and neat Nafion membranes decrease with temperature, the crosslinked HB network yielded a higher storage modulus at elevated temperatures (100 ~ 130 °C).
In order to determine whether or not there is any improvement in the proton transport of the HBPEAc-COOH impregnated membrane over neat Nafion, proton conductivity measurements were conducted by AC impedance fuel cell tests at the humidity levels of 100% RH and 74% RH as a function of temperature. Figure 11(a,b) illustrate log-log plot of complex impedance (Z* = Z′ ‒ iZ″) versus frequency plots obtained for the 90/10 Nafion/HBPEAc-COOH impregnated membrane at 74% RH. The experiment at 100% RH was not feasible beyond 115 °C with the current instrument due to the temperature limitation. The storage impedance (Z′) obtained shows a sigmoidal reduction of impedance values with frequency, whereas the loss impedance (Z″) reveals a corresponding peak around ~20 Hz and slightly shifted to higher frequencies with temperature. The reduction of storage impedance with frequency implies an increasing ionic (proton) resistance of the impregnated electrolyte membrane. It was found that the complex impedance plots of the impregnated membrane at 74% RH showed a similar trend to that at 100% RH, except for slightly different AC impedance values (data not shown).
Next, the Cole-Cole plots were constructed over the frequency range of 0.1 Hz–10 kHz. Figure 11(c) illustrates the Cole-Cole plot of 10 wt % HBPEAc-COOH impregnated membrane at 74%. The first intersection of the plot at the x-axis is denoted as Rs indicating the contact resistance between the electrolyte membrane and the catalyst layer; known as the solution resistance. The diameter of the semi-circular plot, denoted by Rp is taken as the overall cell resistance or polarization resistance [26]. The magnitude of Rp represents the true resistance from which the conductivity value may be evaluated. Conductivity, σ, was then calculated in accordance with Equation (6) as explained in the experimental section. An equivalent circuit of the cell may be represented by a resistor in series with a parallel circuit containing a capacitor and a resistor.
Figure 12 shows the plots of proton conductivity as a function of temperature from 70 to 115 °C at 74% RH of neat Nafion and impregnated membranes. The proton conductivity of neat Nafion is the highest at 80 °C, but gradually declined with increasing of temperature. Since the proton conductivity of Nafion consists of both proton (H+) and hydronium (e.g., H3O+, H5O2+) transport, the loss of water above the boiling temperature can significantly reduce the overall proton conductivity [27] of the hydrophilic clusters. Further increment of temperature results in possible disruption of the percolated ionic channels and the ionic clusters become more isolated thereby reducing the efficiency of proton to conduct.
The impregnated membranes with 10 wt % of HB, on the other hand, exhibits an increasing conductivity trend with temperature afforded by the acidic protons from the pendant carboxylic groups from the HB supramolecules, which gained higher mobility when temperature is raised. The conductivity value of the impregnated membrane increased from 0.040 S cm−1 at 80 °C to 0.056 S cm−1 at 115 °C and at 74% RH, which eventually exceeded that of the neat Nafion (σ = 0.043 S cm−1 at 115 °C and at 74% RH). This observation is not surprising in view of the fact that a sizable portion of the Nafion ionic domain was occupied by the HB networks, which in turn reduced the water uptake. Incorporation of hyperbranched polyesters within the ionic clusters lowers water uptake and therefore lowering conductivity in the impregnated membrane. By reducing the HB amount to 4 wt %, more water molecules can be accommodated with the ionic domains and thus the proton conduction can increase slightly at low temperatures as compared with the 10 wt % sample. At elevated temperature above 100 °C, it shows the proton conductivity behavior that is intermediate between the neat Nafion and the 10 wt % impregnated membrane.
Of particular importance is that the proton conduction of the HBPEAc-COOH impregnated membrane showed an increasing trend that eventually exceeded that of neat Nafion at 110 and 115 °C, even though with only slight improvement in the proton conductivity value. It can be anticipated that the hydrogen bonded carboxylic acids groups present in the ionic clusters/HBPEAc-COOH networks will be ionized with water vapor when the temperature is raised, resulting in free hydronium ions that ultimately promotes the proton conduction process under the minimal water level. Hence, it is reasonable to infer that the proton conducting of the impregnated membrane at elevated temperatures is becoming less dependent on the free water molecules but more on the ionized protons of the carboxylic acid groups. Although the present proton conductivity measurement is limited to 115 °C due to the present instrumental configuration, the impregnated membrane is demonstrated to be thermally more stable up to the experimental temperature of 150 °C as manifested in the previous DMA experiment.
There is a concern that the HB polyester in Nafion membrane may undergo hydrolysis, which is prevalent in most common polyesters. Nafion itself may suffer to some level of membrane deterioration upon prolonged or repeated usage in proton fuel cells due to the formation of peroxide radical originating from the platinum/carbon supported catalyst [28]. HB polyesters might as well be susceptible to such radical attack in the actual proton fuel cell operation. Thus, neat Nafion and HB impregnated membranes were subjected to cyclic AC impedance measurements in proton fuel cell environment by heating and cooling from 30 to 100 °C at 100% RH for a total of 5 cycles. Figure 13 exhibits the heating and cooling cycles of neat Nafion and impregnated membrane and there is a subtle proton conductivity hysteresis between the heating and cooling cycles of neat Nafion (only the first and fifth cycles are shown for clarity) starting from 70 °C up to 100 °C. However, the HB impregnated Nafion reveals little or no change in the proton conductivity up to the 5 cycles tested, i.e., a total of 160 h of operation. Contrary to the general perception on possible hydrolysis of conventional polyester, the present AC measurements on the impregnated membranes show no sign of deterioration in performance in proton conductivity in the repeated cycles under the true proton fuel cell conditions up to 100 °C.
In order to check the potential hydrolysis of polyester, we acquired the FTIR scans on the HB impregnated membranes before and after the cyclic AC impedance measurements. Contrary to the general perception, we did not find any noticeable difference in FTIR spectra before and after the proton fuel cell tests, implying the chemical stability of the HB impregnated membranes up to 100 °C (Figure 14). On the basis of combined cyclic AC impedance measurement and the FTIR investigation, it is reasonable to conclude that the photocured HB polyester network within the Nafion ionic domains appears chemically stable under the conditions of the present proton fuel cell operation at 100 °C for 160 h.
3. Experimental Section
3.1. Materials and Sample Preparation
Nafion 115 membranes with equivalent weight of 1,100 in its original acid form were purchased from Fuel Cell Store. In accordance with the literature procedure [11], as-received Nafion membranes were first pretreated by boiling in 3% hydrogen peroxide (H2O2) solution for 2 h and subsequently rinsed in boiling deionized water. The membranes were then boiled in 0.5 M sulfuric acid (H2SO4) and rinsed again in boiling deionized water (at least 1 h for each step) to remove excess sulfuric acid, and then dried in a vacuum oven and kept in a dessicator prior to each impregnation process.
The HB polyester was synthesized from polyaddition of bisphenol-A-diglycidyl ether (BPGE) with trimesic acid (TMA) and methacrylic acid (MA), by adding cis-1,2,3,6-tetrahydrophthalic anhydride (THPA), triphenylphosphine (TPP) and hydroquinone (HQ) having pendant methacryloyl and carboxyl groups. The molecular weight and polydispersity of neat HBPEAc-COOH in tetrahydrofuran (THF) solution were measured by GPC (Model 1515, Waters) using polystyrene standard. The number average molecular weight and polydispersity were found to be Mn = 6,800 and Mw/Mn = 1.42, respectively. The advantage of the above HB polyester is in its photocuring capability of the acrylate double bonds and the carboxylic acid functionality that affords ionic interactions with its Nafion counterpart. The detailed synthetic scheme of these HB polyesters may be found in a previous paper [10]. ACS grade methanol was purchased from Sigma-Aldrich and used without further purification for dissolving hyperbranched polyester and swelling the ionic domains of Nafion.
Weighed Nafion membranes were pre-swollen in methanol for 24 h and then immersed in the HBPEAc-COOH/methanol solution for 48 h at room temperature. The curing agent (Irgacure 907) in an amount of 3 wt % with respect to HBPEAc-COOH was added to the above solutions. The Nafion to HBPEAc-COOH polyester mass ratios were 98/2, 97/3, 95/5, 90/10 and 80/20. These ratios correspond to the feed compositions, which will be hereafter referred to as ‘feed ratio’. Impregnated membranes were then removed from the HBPEAc-COOH/methanol solution and were gently blotted with tissue paper (e.g., Kimwipes) to remove excess methanol solution on the surface. Subsequently, the impregnated membranes were photocured in a curing chamber (NuLine, model NL22-8C, 90 mW/cm2) at ~85 °C for 5 min, which is slightly above the glass transition temperature (Tg) of the HB polyester. Blotting and curing procedures were conducted in a dark room so as to prevent accidental curing of the membranes. The HBPEAc-COOH impregnated Nafion membranes will be hereafter referred to as ‘impregnated membranes’. These impregnated membranes were kept in a dessicator prior to use.
3.2. Methods
Thermal analyses of neat HBPEAc-COOH before and after photocuring were performed using a differential scanning calorimeter (DSC) using a modulated DSC 2920 (TA Instruments). The recommended amount of 7–10 mg of each sample was encapsulated in aluminum hermatic pans. DSC heating and cooling scans were carried out from 30 to 120 °C at a rate of 10 °C min−1 unless indicated otherwise. The DSC chamber was purged with nitrogen gas at a rate of 80 mL min−1.
1H-13C and 19F-13C cross polarization (CP) and 1H direct polarization (DP) magic angle spinning (MAS) solid-state nuclear magnetic resonance (SSNMR) spectra were collected on a Varian (Model: NMRS 500, 11.7 T) spectrometer operated at 125.62 MHz for 13C and using a Varian narrow-bore triple-resonance T3 MAS NMR probe. Samples were packed into 4 mm zirconia rotors and spun at 10 kHz. The 13C and 1H chemical shifts were referenced to hexamethylbenzene (17.3 ppm; methyl) and adamantane (1.76 ppm). The 1H-13C and 19F-13C CP/MAS data were collected under continuous wave (CW) proton and TPPM fluorine decoupling, respectively. A 90° pulse width of 4 μs was used for all nuclei. Recycle delays of 2, 2.5 and 1 s were used for 1H-13C CP, 19F-13C CP and 1H DP experiments with contact times of 3.5 and 1.5 ms for the 1H-13C and 19F-13C CP experiments. For the 1H spectra, 32 transients were collected. Samples of neat Nafion-acid and impregnated membrane were stored in a dessicator and only exposed to ambient atmosphere while loading. The membranes were cut into a rectangular shape and rolled to fit into the rotors.
Fourier transform infrared (FTIR) spectra were collected in the attenuated total reflected mode (ATR) on a Nicolet 380 (Thermo Scientific) spectrometer with an average of 32 scans and a spectral resolution of 4 cm−1 at ~100 °C. Prior to the spectra collection, the membrane samples were equilibrated at 100 °C for about 10 min to eliminate possible moisture absorption.
Quantification of the estimated amount of HBPEAc-COOH in the impregnated membranes was done by integrating the area under the FTIR curves within the wavenumber ranges of 1,670–1,770 cm−1 and 1,550–1,450 cm−1, which correspond to the carbonyl group and C=C aromatic stretching peaks, respectively. The area under the FTIR absorption peak was normalized by that of pure HB polyester. The estimated amount of HB polyester was calculated by multiplying the percentage of HBPEAc-COOH present in membrane with the initial amount in feed and can be calculated using Equations (1) and (2) as described below;
Prior to the water uptake measurements, neat Nafion and HBPEAc-COOH impregnated Nafion membranes were dried in a vacuum oven until a constant weight was reached, which was taken as the mass in the dry state. All membranes were immersed in 10 mL deionized water at room temperature and equilibrated for 72 h for water uptake measurements. Membranes were then removed and blotted with dry tissue papers (Kimwipes) and the mass in wet state was recorded. Water uptake in percentage and the number of water molecules per mole of sulfonic group, λ, can be determined using Equations (3) and (4) as follows;
Ion exchange capacity (IEC) of impregnated membranes and neat Nafion were conducted using titration method where weighed membrane samples were soaked in sodium chloride (NaCl) solution for approximately 120 h to ensure complete conversion of proton to sodium cations. Solutions were then titrated with standardized 0.01 N sodium hydroxide (NaOH) using a phenolphthalein indicator to the end point. IEC values in meq g−1 were calculated using the volume of sodium hydroxide (NaOH) used to neutralize the solution with equation below;
Dynamic mechanical analyses (DMA) were measured in sinusoidal tension mode using a Pyris Diamond DMA (Perkin Elmer) equipped with a liquid nitrogen cooling controller (Seiko Equipment). Dried neat Nafion and impregnated membranes were cooled down to ‒140 °C and maintained isothermally for 5 min, then heated up to 200 °C at a heating rate of 1 °C min−1. The frequency of the dynamic mechanical measurements was fixed at 1 Hz and measurements were carried out under the nitrogen environment.
Proton conductivity characterization of neat Nafion 115 and impregnated membranes were conducted using the AC impedance fuel cell device (Scribner, Model 850e Multi Range) equipped with adjustable reactant humidifier unit. Hydrogen (H2) and compressed air were used as reactants with flow rate of 0.2 L min−1. Contact cell dimension used was 5 cm2 and membranes were tested at frequency range of 0.1 Hz–10 kHz. Measurements were conducted at two different relative humidity (RH) levels, i.e., 100% RH and 74% RH ranging from 30 to 110 °C. Complex impedance data obtained were analyzed using the ZView program. Conductivity value can be calculated as follows;
4. Conclusions
We have successfully incorporated large polymer molecules into the Nafion ionic domains via impregnation of hyperbranched supramolecules and subsequent photo-crosslinking in-situ. The FTIR studies suggested the occurrence of complexation, presumably inter-species hydrogen bonding between the carboxylic group of HB supramolecules and the sulfonate group of Nafion through bound water molecules. HBPEAc-COOH impregnated Nafion membranes exhibited lower swelling with improved dimensional stability in the presence of polar solvents. It was found that impregnating Nafion with HB solution increases the IEC values and therefore the proton density within the ionic clusters increases. The enhancement of IEC values in impregnated membranes can be attributed to the presence of collective protons from carboxylates in HB together with the primary protons of sulfonate groups in Nafion. Another important finding is the improved thermal stability of HBPEAc-COOH impregnated Nafion, showing the movement of the mechanical α-relaxation to a higher temperature of 130 °C relative to 100 °C of the neat Nafion as well as the increase in the storage modulus. It may concluded that the impregnated membrane reveals an increasing trend of proton conductivity with temperature as opposed to the neat Nafion that shows the declining trend at elevated temperatures. Moreover, the present AC measurements on the impregnated membranes show no sign of deterioration in performance under the true proton fuel cell operating conditions in the repeated cycles up to 100 °C, implying the durability of these impregnated membranes. Further, FTIR spectra of the HB impregnated membrane shows virtually unchanged before and after the cyclic AC impedance tests, which eliminates some doubts by others on possible hydrolysis of the HB polyesters at least for the duration (i.e., 160 h) of the AC cyclic tests at 100 °C under the actual proton fuel cell conditions.














Acknowledgments
The authors are indebted to Robert Weiss and his group members, Emmanuel Pitia and Nathinee Srinate, for their invaluable suggestions and assistance in the operation of the AC impedance proton fuel cell device.
References
- Grot, W.G.F. Discovery and development of Nafion perfluorinated membranes. Chem. Ind. 1985, 9, 647–649. [Google Scholar]
- Grot, W.G.F. Perfluorinated ion exchange polymers and their use in research and industry. Macromol. Symp. 1994, 82, 161–172. [Google Scholar]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Miyake, N.; Wainright, J.S.; Savinell, R.F. Evaluation of a sol-gel derived nafion/silica hybrid membrane for proton electrolyte membrane fuel cell applications: I. Proton conductivity and water content. J. Electrochem. Soc. 2001, 148, A898–A904. [Google Scholar]
- DeLuca, N.W.; Elabd, Y.A. Nafion®/poly(vinyl alcohol) blends: Effect of composition and annealing temperature on transport properties. J. Membr. Sci. 2006, 282, 217–224. [Google Scholar]
- Doyle, M.; Choi, S.K.; Proulx, G. High-temperature proton conducting membranes based on perfluorinated ionomer membrane-ionic liquid composites. J. Electrochem. Soc. 2000, 147, 34–37. [Google Scholar]
- Schmidt, C.; Glück, T.; Schmidt-Naake, G. Modification of nafion membranes by impregnation with ionic liquids. Chem. Eng. Technol. 2008, 31, 13–22. [Google Scholar]
- Mistry, M.K.; Subianto, S.; Choudhury, N.R.; Dutta, N.K. Interfacial interactions in aprotic ionic liquid based protonic membrane and its correlation with high temperature conductivity and thermal properties. Langmuir 2009, 25, 9240–9251. [Google Scholar]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. In situ modification of Nafion® membranes with phospho-silicate for improved water retention and proton conduction. J. Membr. Sci. 2009, 333, 50–58. [Google Scholar]
- Maruyama, K.; Kudo, H.; Ikehara, T.; Ito, N.; Nishikubo, T. Synthesis of photocrosslinkable hyperbranched polyesters and their film properties. J. Polym. Sci. Part A: Polym. Chem. 2005, 43, 4642–4653. [Google Scholar]
- Zawodzinski, T.A.; Springer, T.E.; Davey, J.; Jestel, R.; Lopez, C.; Valerio, J.; Gottesfeld, S. Water uptake by and transport through Nafion® 117 membranes. J. Electrochem. Soc. 1993, 140, 1981–1985. [Google Scholar]
- Liu, S.-F.; Schmidt-Rohr, K. High-resolution solid-state 13C NMR of fluoropolymers. Macromolecules 2001, 34, 8416–8418. [Google Scholar]
- Chen, Q.; Schmidt-Rohr, K. 19F and 13C NMR signal assignment and analysis in a perfluorinated ionomer (nafion) by two-dimensional solid-state NMR. Macromolecules 2004, 37, 5995–6003. [Google Scholar]
- Takasaki, M.; Kimura, K.; Kawaguchi, K.; Abe, A.; Katagiri, G. Structural analysis of a perfluorosulfonate ionomer in solution by 19F and 13C NMR. Macromolecules 2005, 38, 6031–6037. [Google Scholar]
- Bunce, N.J.; Sondheimer, S.J.; Fyfe, C.A. Proton NMR method for the quantitative determination of the water content of the polymeric fluorosulfonic acid Nafion-H. Macromolecules 1986, 19, 333–339. [Google Scholar]
- Batamack, P.; Fraissard, J. Interaction of the perfluorinated polymer Nafion-H with water studied by proton broad-line NMR at 4 K and MAS NMR at room temperature. Catal. Lett. 1995, 35, 135–142. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; Wolfman-Robichaud, S., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; Chapter 2; pp. 72–126. [Google Scholar]
- Ostrowska, J.; Narębska, A. Infrared study of hydration and association of functional groups in a perfluorinated Nafion membrane, Part 1. Colloid Polym. Sci. 1983, 261, 93–98. [Google Scholar]
- Falk, M. Perfluorinated Ionomer Membranes; Eisenberg, A., Yeager, H.L., Eds.; ACS Symp. Ser. 180; American Chemical Society: Washington, DC, USA, 1982; Chapter 8; pp. 139–171. [Google Scholar]
- Yeo, R.S. Swelling studies of perfluorinated ionomer membranes. J. Appl. Polym. Sci. 1986, 32, 5733–5741. [Google Scholar]
- Elliott, J.A.; Hanna, S.; Elliott, A.M.S.; Cooley, G.E. The swelling behaviour of perfluorinated ionomer membranes in ethanol/water mixtures. Polymer 2001, 42, 2251–2253. [Google Scholar]
- Hinatsu, J.T.; Mizuhata, M.; Takenaka, H. Water uptake of perfluorosulfonic acid membranes from liquid water and water vapor. J. Electrochem. Soc. 1994, 141, 1493–1498. [Google Scholar]
- Paddison, S.J. Proton conduction mechanisms at low degrees of hydration in sulfonic acid-based polymer electrolyte membranes. Annu. Rev. Mater. Res. 2003, 33, 289–319. [Google Scholar]
- Kyu, T.; Hashiyama, M.; Eisenberg, A. Dynamic mechanical studies of partially ionized and neutralized Nafion polymers. Can. J. Chem. 1983, 61, 680–687. [Google Scholar]
- Kyu, T.; Eisenberg, A. Underwater stress relaxation studies of Nafion (perfluorosulfonate) ionomer membranes. J. Polym. Sci. Polym. Symp. 1984, 71, 203–219. [Google Scholar]
- Yuan, X.Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 39–93. [Google Scholar]
- Anantaraman, A.V.; Gardner, C.L. Studies on ion-exchange membranes. Part 1. Effect of humidity on the conductivity of Nafion®. J. Electroanal.Chem. 1996, 414, 115–120. [Google Scholar]
- Endoh, E.; Terazono, S.; Widjaja, H.; Takimoto, Y. Degradation study of MEA for PEMFCs under low humidity conditions. Electrochem. Solid State Lett. 2004, 7, A209–A211. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nazir, N.A.; Kyu, T.; Reinsel, A.M.; Espe, M.; Nosaka, M.; Kudo, H.; Nishikubo, T. Incorporation of Hyperbranched Supramolecules into Nafion Ionic Domains via Impregnation and In-Situ Photopolymerization. Polymers 2011, 3, 2018-2038. https://doi.org/10.3390/polym3042018
Nazir NA, Kyu T, Reinsel AM, Espe M, Nosaka M, Kudo H, Nishikubo T. Incorporation of Hyperbranched Supramolecules into Nafion Ionic Domains via Impregnation and In-Situ Photopolymerization. Polymers. 2011; 3(4):2018-2038. https://doi.org/10.3390/polym3042018
Chicago/Turabian StyleNazir, Nadzrinahamin A., Thein Kyu, Anna M. Reinsel, Matthew Espe, Mami Nosaka, Hiruto Kudo, and Tadatomi Nishikubo. 2011. "Incorporation of Hyperbranched Supramolecules into Nafion Ionic Domains via Impregnation and In-Situ Photopolymerization" Polymers 3, no. 4: 2018-2038. https://doi.org/10.3390/polym3042018
APA StyleNazir, N. A., Kyu, T., Reinsel, A. M., Espe, M., Nosaka, M., Kudo, H., & Nishikubo, T. (2011). Incorporation of Hyperbranched Supramolecules into Nafion Ionic Domains via Impregnation and In-Situ Photopolymerization. Polymers, 3(4), 2018-2038. https://doi.org/10.3390/polym3042018