Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling
Abstract
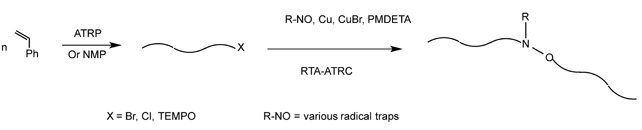
:1. Introduction

2. Experimental Section
2.1. Materials
2.2. PSBr Synthesis
2.3. PSCl Synthesis
2.4. PS-TEMPO Synthesis
2.5. Coupling of PSBr by RTA-ATRC
2.6. Coupling of PSCl by RTA-ATRC
2.7. Coupling of PS-TEMPO by RTA-ATRC
2.8. Synthesis of 4,4'-Diphenylmethanedicarbamic Acid Di(1-oxy-2,2,6,6-tetramethylpiperidin-4-yl) Ester (DNX, Scheme 2)
2.9. Characterization
3. Results and Discussion
3.1. Role of the Radical Trap in RTA-ATRC Reactions of PSBr Chains
| PSBr Precursor a | RTA-ATRC and ATRC Products b | |||||||
|---|---|---|---|---|---|---|---|---|
| Trial | Mn c | Mp e | Đ f | Coupling Trap d | Mn c | Mp e | Đ f | [PSBr]:[CuBr]:[Cu0]:[PMDETA]:[Radical Trap] |
| 1 | 3,750 | 4,000 | 1.09 | - | 6,600 | 8,600 | 1.19 | 1:5:5:10:- |
| 2 | 3,750 | 4,000 | 1.09 | DNX | 7,200 | 10,750 | 1.24 | 1:5:5:10:0.6 |
| 3 | 3,750 | 4,000 | 1.09 | MNP | 6,850 | 8,700 | 1.16 | 1:5:5:10:0.6 |
| 4 | 7,650 | 8,000 | 1.15 | ATRC | 13,200 | 17,600 | 1.35 | 1:3:3:6:- |
| 5 | 7,650 | 8,000 | 1.15 | DNX | 12,100 | 18,700 | 1.33 | 1:3:3:6:0.6 |
| 6 | 7,650 | 8,000 | 1.15 | MNP | 13,350 | 16,200 | 1.25 | 1:3:3:6:0.6 |
| 7 | 6,450 | 6,800 | 1.08 | DMNA | 7,450 | 6,830 | 1.18 | 1:3:3:6:0.6 |
| 8 | 6,450 | 6,800 | 1.08 | NDPA | 9,050 | 13,700 | 1.21 | 1:3:3:6:0.6 |
| 9 | 6,450 | 6,800 | 1.08 | NBz | 10,800 | 13,700 | 1.16 | 1:3:3:6:0.6 |

3.2. Coupling PSCl Chains Using ATRC and RTA-ATRC
| PSCl Precursor a | RTA-ATRC and ATRC Products b | |||||||
|---|---|---|---|---|---|---|---|---|
| Trial | Mn c | Mp e | Đ f | Coupling Trap d | Mn c | Mpe | Đ f | [PSCl]:[CuCl]:[Cu0]:[PMDETA]:[Radical Trap] |
| 1 | 6,700 | 9,400 | 1.35 | - | 7,400 | 10,400 | 1.41 | 1:3:3:6:- |
| 2 | 6,700 | 9,400 | 1.35 | DNX | 8,000 | 11,100 | 1.44 | 1:3:3:6:0.6 |
| 3 | 6,700 | 9,400 | 1.35 | MNP | 9,100 | 14,450 | 1.45 | 1:3:3:6:0.6 |
| 4 | 6,450 | 8,050 | 1.24 | DMNA | 5,200 | 8,100 | 1.45 | 1:3:3:6:0.6 |
| 5 | 6,450 | 8,050 | 1.24 | NDPA | 5,750 | 8,200 | 1.41 | 1:3:3:6:0.6 |
| 6 | 6,450 | 8,050 | 1.24 | MNP | 6,650 | 13,450 | 1.66 | 1:3:3:6:0.6 |
| 7 | 6,450 | 8,050 | 1.24 | NBz | 7,150 | 13,800 | 1.72 | 1:3:3:6:0.6 |

3.3. Coupling PS-TEMPO Chains Using RTA-ATRC-Type Reactions

| PS-TEMPO Precursor a | RTA-ATRC Products b | ||||||
|---|---|---|---|---|---|---|---|
| Trial | Mn c | Mp d | Đ e | Radical Trap f | Mn c | Mp d | Đ e |
| 1 | 7,700 | 9,000 | 1.20 | MNP | 8,600 | 8,850 | 1.28 |
| 2 | 7,700 | 9,000 | 1.20 | PBN | 8,300 | 8,850 | 1.25 |
| 4 | 7,700 | 9,000 | 1.20 | NDPA | 7,800 | 8,750 | 1.21 |
| 5 | 11,800 | 16,000 | 1.34 | MNP | 11,600 | 15,550 | 1.34 |
| 6 | 11,800 | 16,000 | 1.34 | PBN | 11,900 | 16,100 | 1.30 |
| 7 | 11,800 | 16,000 | 1.34 | NDPA | 12,550 | 16,850 | 1.34 |
3.4. Kinetic Comparison between ATRC and RTA-ATRC in Coupling PSBr
| Sample # | ATRC Product a | RTA-ATRC Product b | ||||||
|---|---|---|---|---|---|---|---|---|
| Time of Sample Taken c (min) | Mn d | Mp e | Đ f | Time of Sample Taken c (min) | Mn d | Mp e | Đ f | |
| 1 | 10 | 4,650 | 5,100 | 1.24 | 2 | 4,550 | 5,000 | 1.23 |
| 2 | 20 | 5,100 | 5,150 | 1.31 | 5 | 4,700 | 5,050 | 1.34 |
| 3 | 25 | 5,350 | 9,950 | 1.37 | 7 | 5,000 | 9,650 | 1.34 |
| 4 | 30 | 5,650 | 9,850 | 1.37 | 10 | 5,250 | 9,800 | 1.37 |
| 5 | 40 | 5,950 | 10,050 | 1.41 | 15 | 5,700 | 9,950 | 1.37 |
| 6 | 50 | 6,500 | 10,000 | 1.36 | 20 | 5,850 | 10,000 | 1.39 |
| 7 | 60 | 5,700 | 9,900 | 1.47 | 25 | 6,000 | 10,000 | 1.39 |
| 8 | - | - | - | - | 30 | 5,650 | 10,000 | 1.46 |
| 9 | - | - | - | - | 40 | 5,750 | 9,950 | 1.46 |
| 10 | - | - | - | - | 50 | 5,700 | 10,000 | 1.47 |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butcher, W.E.; Radzinski, S.C.; Tillman, E.S. Selective formation of diblock copolymers using radical trap-assisted atom transfer radical coupling. J. Poly. Sci. A Polym. Chem. 2013, 51, 3619–3626. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Coyne, W.E.; Myers, K.D.; Tillman, E.S. One pot, two step sequence converting Atom transfer radical polymerization directly to radical trap-assisted atom transfer radical coupling. Polymer 2013, 54, 5560–5567. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q. Degradable multisegmented polymers synthesized by consecutive radical addition-coupling reaction of α,ω-macrobiradicals and nitroso compound. J. Poly. Sci. A Polym. Chem. 2011, 49, 612–618. [Google Scholar]
- Sarbu, T.; Lin, K.; Ell, J.; Siegwart, D.; Spanswick, J.; Matyjaszewski, K. Polystyrene with designed molecular weight distribution by atom transfer radical coupling. Macromolecules 2004, 37, 3120–3127. [Google Scholar] [CrossRef]
- Atanu, K.; Chatterjee, D.P.; Layek, R.K.; Nandi, A.K. Coupled atom transfer radical coupling and atom transfer radical polymerization approach for controlled grafting from poly(vinylidene fluoride) backbone. J. Poly. Sci. A Polym. Chem. 2014, 52, 995–1008. [Google Scholar] [CrossRef]
- Wang, G.W.; Huang, J.L. Versatility of radical coupling in construction of topololgical polymers. Polym. Chem. 2014, 5, 277–308. [Google Scholar] [CrossRef]
- Valente, C.J.; Schellenberger, A.M.; Tillman, E.S. Dimerization of poly(methyl methacrylate) chains using radical trap-assisted atom transfer radical coupling. Macromolecules 2014, 47, 2226–2232. [Google Scholar] [CrossRef]
- Yurteri, S.; Cianga, I.; Yagci, Y. Synthesis and characterization of α,ω-telechelic polymers by atom transfer radical polymerization and coupling processes. Macromol. Chem. Phys. 2003, 204, 1771–1783. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.Y.; Spanswick, J.; Gil, R.R.; Siegwart, D.J.; Matyjaszewski, K. Synthesis of hydroxy-telechelic poly(methyl acrylate) and polystyrene by atom transfer radical coupling. Macromolecules 2004, 37, 9694–9700. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/“living” radical polymerization atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine)ruthenium(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar]
- Patten, T.E.; Matyjaszewski, K. Copper(I)-catalyzed atom transfer radical polymerization. Acc. Chem. Res. 1999, 32, 895–903. [Google Scholar]
- Tang, H.; Arulsamy, N.; Radosz, M.; Shen, Y.; Tsarevsky, N.V.; Braunecker, W.A.; Tang, W.; Matyjaszewski, K. Highly active copper-based catalyst for atom transfer radical polymerization. J. Am. Chem. Soc. 2006, 128, 16277–16285. [Google Scholar] [CrossRef] [PubMed]
- Voter, A.F.; Tillman, E.S. An easy and efficient route to macrocyclic polymers via intramolecular radical-radical coupling of chain ends. Macromolecules 2010, 43, 10304–10310. [Google Scholar]
- Voter, A.F.; Tillman, E.S.; Findeis, P.; Radzinski, S.C. Synthesis of macrocyclic polymers formed via intramolecular radical trap-assisted atom transfer radical coupling. ACS Macro Lett. 2012, 1, 1066–1070. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Tillman, E.S. Polymerization of styrene and cyclization to macrocyclic polystyrene in a one-pot, two-step sequence. React. Funct. Polym. 2014, 80, 9–14. [Google Scholar] [CrossRef]
- Jiang, X.Z.; Vamvakaki, M.; Narain, R. Copper-catalyzed bimolecular coupling of α,ω-dibromide-functionalized poly(γ-caprolactone). Macromolecules 2010, 43, 3228–3232. [Google Scholar] [CrossRef]
- Huang, C.F.; Ohta, Y.; Yokoyama, A.; Yokozawa, H. Efficient low-temperature atom transfer radical coupling and its application to synthesis of well-defined symmetrical polybenzamides. Macromolecules 2011, 44, 4140–4148. [Google Scholar] [CrossRef]
- Greene, A.C.; Grubbs, R.B. Nitroxide-mediated polymerization of methyl methacrylate and styrene with new alkoxyamines from 4-nitrophenyl 2-methylpropionat-2-yl radicals. Macromolecules 2010, 43, 10320–10325. [Google Scholar] [CrossRef]
- Buback, M.; Egorov, M.; Gilbert, R.G.; Kaminsky, V.; Olaj, O.F.; Russell, G.T.; Vana, P.; Zifferer, G. Critically evaluated termination rate coefficients for free-radical polymerization, 1. The current situation. Macromol. Chem. Phys. 2002, 203, 2570–2582. [Google Scholar] [CrossRef]
- Lizotte, J.R.; Anderson, S.G.; Long, T.E. Novel dinitroxide mediating agent for stable free-radical polymerization. J. Polym. Sci. A Polym. Chem. 2004, 42, 1547–1556. [Google Scholar] [CrossRef]
- Domingues, K.D.; Tillman, E.S. Radical-radical coupling of polystyrene chains using AGET ATRP. J. Poly. Sci. A Polym. Chem. 2010, 48, 5737–5745. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [PubMed]
- Hawker, C.J.; Barclay, G.G.; Dao, J. Radical crossover in nitroxide mediated “living” free radical polymerizations. J. Am. Chem. Soc. 1996, 46, 11467–11471. [Google Scholar] [CrossRef]
- Listigovers, N.A.; Georges, M.K.; Odell, P.G.; Keoshkerian, B. Narrow-polydispersity diblock and triblock copolymers of alkyl acrylates by a “living” stable free radical polymerization. Macromolecules 1996, 29, 8992–8993. [Google Scholar]
- Fukuda, T.; Terauchi, T.; Goto, A.; Ohno, K.; Tsujii, Y.; Miyamoto, T.; Kobatake, S.; Yamada, B. Mechanisms and kinetics of nitroxide-controlledf Radical polymerization. Macromolecules 1996, 29, 6393–6398. [Google Scholar]
- Radzinski, S.C.; Tillman, E.S. Trapping polystyrene radicals with nitrones: Synthesis of polymers with mid-chain alkoxyamine functionality. Polymer 2011, 52, 6003–6010. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnicom, E.M.; Abruzzese, J.A.; Sidibe, Y.; Myers, K.D.; Tillman, E.S. Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling. Polymers 2014, 6, 2737-2751. https://doi.org/10.3390/polym6112737
Carnicom EM, Abruzzese JA, Sidibe Y, Myers KD, Tillman ES. Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling. Polymers. 2014; 6(11):2737-2751. https://doi.org/10.3390/polym6112737
Chicago/Turabian StyleCarnicom, Elizabeth M., Jessica A. Abruzzese, Yacouba Sidibe, Kenneth D. Myers, and Eric S. Tillman. 2014. "Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling" Polymers 6, no. 11: 2737-2751. https://doi.org/10.3390/polym6112737
APA StyleCarnicom, E. M., Abruzzese, J. A., Sidibe, Y., Myers, K. D., & Tillman, E. S. (2014). Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling. Polymers, 6(11), 2737-2751. https://doi.org/10.3390/polym6112737