Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Metal Sulfides/PMMA Nanocomposites
2.3. Characterization
3. Results and Discussion
3.1. Infrared Spectra Studies

3.2. X-ray Diffraction Studies

3.3. Thermogravimetric Analysis of the Metal Sulfides/PMMA Nanocomposites

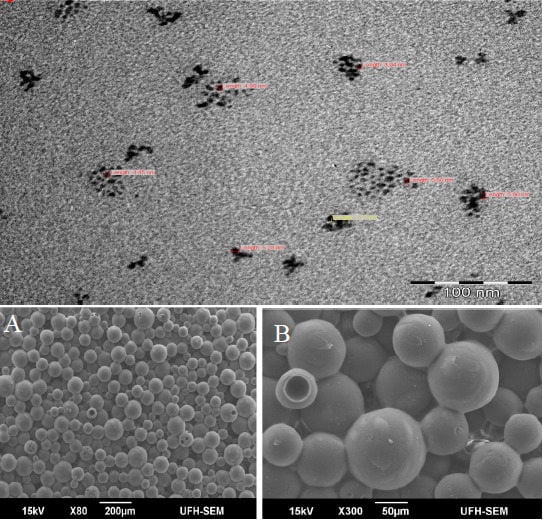
3.4. SEM and EDX Studies of the Nanocomposites

3.5. TEM Studies of Nanocomposites

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carotenuto, G.; Nicolais, L.; Perlo, P.; Martorana, B. Method of production of polymer/metal or metal sulphide composites which uses metal mercaptides. U.S. Patent 7329700, 20 December 2014. [Google Scholar]
- Capezzuto, F.; Carotenuto, G.; Antolini, F.; Burresi, E.; Palomba, M.; Perlo, P. New fluorescent polymeric nanocomposites synthesized by antimony dodecyl-mercaptide thermolysis in polymer. Express Poly. Lett. 2009, 3, 219–225. [Google Scholar] [CrossRef]
- Caseri, W. Nanocomposites of polymers and metals or semiconductors: Historical background and optical properties. Macromol. Rap. Commun. 2000, 21, 705–722. [Google Scholar] [CrossRef]
- Mthethwa, T.P.; Moloto, M.J.; de Vries, A.; Matabola, K.P. Properties of electrospun CdS and CdSe filled poly(methyl methacrylate) (PMMA) nanofibres. Mater. Res. Bull. 2011, 46, 569–575. [Google Scholar]
- Dixit, M.; Gupta, S.; Mathur, V.; Rathore, K.S.; Sharma, K.; Saxena, N.S. Study of glass transition temperature of PMMA and CdS-PMMA composite. Chalco. Lett. 2009, 6, 131–136. [Google Scholar]
- Jeon, I.Y.; Baek, J.B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar]
- Kaltenhauser, V.; Rath, T.; Haas, W.; Torvisco, A.; Muller, S.K.; Friedel, B.; Kunert, B.; Saf, R.; Hofer, F.; Trimmel, G. Bismuth sulphide-polymer nanocomposites from a highly soluble bismuth xanthate precursor. J. Mater. Chem. C 2013, 1, 7825–7832. [Google Scholar]
- Zhang, C.Q.; Sun, J.; Wang, W.; Yang, Q.B.; Li, Y.X.; Du, J.S. Facile method to prepare metal sulfide (Ag2S, CuS, PbS) nanoparticles grown on surface of polyacrylonitrile nanofibre and their optical properties. Chem. Res. Chin. Univ. 2012, 28, 534–538. [Google Scholar]
- Ranjbar, M.; Yousefi, M.; Nozari, R.; Sheshmani, S. Synthesis and characterization of cadmium-thioacetamide nanocomposites using a facile sonochemical approach: A precursor for producing Cds nanoparticles via thermal decomposition. Int. J. Nanosci. Nanotechnol. 2013, 9, 203–212. [Google Scholar]
- Kurahatti, R.V.; Surendranathan, A.O.; Kori, S.A.; Singh, N.; Kumar, A.V.R.; Srivastava, S. Defence applications of polymer nanocomposites. Def. Sci. J. 2010, 60, 551–563. [Google Scholar]
- Jayanthi, K.; Chawla, S.; Chander, H.; Haranath, D. Structural, optical and photoluminescence properties of ZnS: Cu nanoparticle thin films as a function of dopant concentration and quantum confinement effect. Cryst. Res. Technol. 2007, 42, 976–982. [Google Scholar]
- O’Brien, P.; Peakett, N.L. Nanocrystalline semiconductors: Synthesis, properties, and perspectives. Chem. Mater. 2001, 13, 3843–3858. [Google Scholar] [CrossRef]
- Singh, C.P.; Bindra, K.S.; Oak, S.M. Nonlinear optical studies in semiconductor-doped glasses under femtosecond pulse excitation. Pramana J. Phys. 2010, 75, 1169–1173. [Google Scholar]
- Khaorapapong, N.; Ontam, A.; Ogawa, M. Very slow formation of copper sulfide and cobalt sulfide nanoparticles in montmorillonite. Appl. Clay Sci. 2011, 51, 82–186. [Google Scholar]
- Indulal, C.R.; Kumar, G.S.; Vaidyan, A.V.; Raveendran, R. Oxide nanostructures: Characterisations and optical bandgap evaluations of cobalt manganese and nickel at different temperatures. J. Nano Electron. Phys. 2011, 3, 170–178. [Google Scholar]
- Sinkó, K.; Szabó, G.; Zrínyi, M. Liquid-phase synthesis of cobalt oxide nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 1–9. [Google Scholar]
- Ezema, F.I. Preparation and optical characterization of chemical bath deposited CdCoS2 Thin Films. J. Appl. Sci. 2006, 6, 1827–1832. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ajibade, P.A. ZnS, CdS and HgS nanoparticles via alkyl-phenyl dithiocarbamate complexes as single source precursors. Int. J. Mol. Sci. 2011, 12, 5538–5551. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Chen, W.W.; Hu, X.Y. Controllable synthesis and optical properties of Zn-doped CdS nanorods from single-source molecular precursors. Cryst. Growth Des. 2007, 7, 580–586. [Google Scholar]
- Plante, I.J.L.; Zeid, T.W.; Yang, P.; Mokari, T. Synthesis of metal sulfide nanomaterials via thermal decomposition of single-source precursors. J. Mater. Chem. 2010, 20, 6612–6617. [Google Scholar]
- Prabhu1, S.G.; Pattabi, B.M. Incorporation of acetoacetanilide crystals in host PMMA polymer matrix and characterizations of the hybrid composite. J. Mine. Mater. Charact. Eng. 2012, 11, 519–527. [Google Scholar]
- Agrawal, S.; Patidar, D.; Saxena, N.S. Glass transition temperature and thermal stability of ZnS/PMMA nanocomposites. Phase Trans. 2011, 84, 888–900. [Google Scholar]
- Nicolais, L.F.; Carotenuto, G. Synthesis of polymer-embedded metal, semimetal, or sulfide clusters by thermolysis of mercaptide molecules dissolved in polymers. Recent Pat. Mater. Sci. 2008, 1, 1–11. [Google Scholar] [CrossRef]
- Agarwal, S.; Patidar, D.; Saxena, N.S. Effect of ZnS nanofiller and temperature on mechanical properties of poly (methyl methacrylate). J. Appl. Polym. Sci. 2012, 123, 2431–2438. [Google Scholar]
- Li, Z.; Zhang, J.; Du, J.; Mu, T.; Liu, Z.; Chen, J.; Han, B. Preparation of cadmium sulfide/poly(methyl methacrylate) composites by precipitation with compressed CO2. J. Appl. Polym. Sci. 2004, 94, 1643–1648. [Google Scholar] [CrossRef]
- Guan, C.; Lu, C.; Cheng, Y.; Song, S.; Yang, B. A facile one-pot route to transparent polymer nanocomposites with high ZnS nanophase contents via in situ bulk polymerization. J. Mater. Chem. 2009, 19, 617–621. [Google Scholar]
- He, R.; Qian, X.F.; Yin, J.; Bian, L.J.; Xi, H.A.; Zhu, Z.K. In situ synthesis of CdS/PVK nanocomposites and their optical properties. Mater. Lett. 2003, 57, 1351–1354. [Google Scholar] [CrossRef]
- Yao, J.; Zhao, G.; Wang, D.; Han, G. Solvothermal synthesis and characterization of CdS nanowires/PVA composite films. Mater. Lett. 2005, 59, 3652–3655. [Google Scholar] [CrossRef]
- Borah, J.P.; Sarma, K.C. Photo-physical properties of ZnS/PVA nanocrystals. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 891–898. [Google Scholar]
- Bhaiswar, J.B.; Salunkhe, M.Y.; Dongre, S.P. Synthesis, characterization and thermal, electrical study of CdS-polyaniline nanocomposite via oxidation polymerization. Int. J. Sci. Res. Public. 2013, 3, 1–4. [Google Scholar]
- Lee, H.L.; Mohammed, I.A.; Belmahi, M.; Assouar, M.B.; Rinnert, H.; Alnot, M. Thermal and optical properties of CdS nanoparticles in thermotropic liquid crystal monomers. Materials 2010, 3, 2069–2086. [Google Scholar]
- Mehta, S.K.; Kumar, S.; Chaudhary, S.; Bhasin, K.K. Nucleation and growth of surfactant-passivated CdS and HgS nanoparticles: Time-dependent absorption and luminescence profiles. Nanoscale 2010, 2, 145–152. [Google Scholar]
- Oshal, F.; Mossalayi, H. Effect of matrices on size and morphology of HgS nanoparticles. Der Pharma Chemica 2010, 2, 33–37. [Google Scholar]
- Jang, J.; Kim, S.; Lee, K.J. Fabrication of CdS/PMMA core/shell nanoparticles by dispersion mediated interfacial polymerization. Chem. Commun. 2007, 2689–2691. [Google Scholar] [CrossRef]
- Kuljanin, J.; Marinović-Cincović, M.; Stojanović, Z.; Krklješ, A.; Abazović, N.D.; Comor, M.I. Thermal degradation kinetics of polystyrene/cadmium sulfide composites. Polym. Degrad. Stab. 2009, 94, 891–897. [Google Scholar]
- Marimuthu, G.; Ramalingam, K.; Rizzoli, C.; Arivanandhan, M. Solvothermal preparation of nano-b-HgS from a precursor, bis(dibenzyldithiocarbamato)mercury(II). J. Nanopart. Res. 2012, 14, 1–11. [Google Scholar]
- Tamrakar, R.; Ramrakhiani, M.; Chandra, B.P. Effect of capping agent concentration on photophysical properties of zinc sulfide nanocrystals. Open Nanosc. J. 2008, 2, 12–16. [Google Scholar]
- Lee, S.J.; Kim, K.N.; Bae, P.K.; Chang, J.H.; Kim, Y.R.; Park, J.K. Sonication treatment of CdTe/CdS semiconductor nanocrystals and their bio-application. Chem. Commun. 2008, 43, 5574–5576. [Google Scholar]
- Wei, S.; Sampathi, J.; Guo, Z.; Anumandla, N.; Rutman, D.; Kucknoor, A.; James, L.; Wang, A. Nanoporous poly(methyl methacrylate)-quantum dots nanocomposite fibers toward biomedical applications. Polymer 2011, 52, 5817–5829. [Google Scholar]
- Liu, S.; Zhang, H.; Swihart, M.T. Spray pyrolysis synthesis of ZnS nanoparticles from a single-source precursor. Nanotech. 2009, 20, 1–8. [Google Scholar]
- Jothi, N.S.N.; Gunaseelan, R.; Raj, T.M.; Sagayaraj, P. Investigation on mild condition preparation and structural, optical and thermal properties of PVP capped CdS nanoparticles. Arch. Appl. Sci. Res. 2012, 4, 1723–1730. [Google Scholar]
- Barman, B.; Sarma, K.C. Luminescence properties of ZnS quantum dots embedded in polymer matrix. Chalc. Lett. 2011, 8, 171–176. [Google Scholar]
- Sharma, R. Optical studies of CdS:Mn nanoparticles. Luminescence 2012, 27, 501–504. [Google Scholar]
- Sharma, M.; Kumara, S.; Pandey, O.P. Photo-physical and morphological studies of organically passivated core-shell ZnS nanoparticles. Dig. J. Nanomater. Biostruct. 2008, 3, 189–197. [Google Scholar]
- Tomar, A.K.; Mahendia, S.; Kumar, S. Structural characterization of PMMA blended with chemically synthesized PAni. Adv. Appl. Sci. Res. 2011, 2, 65–71. [Google Scholar]
- Hashmi, L.; Sana, P.; Malik, M.M.; Siddiqui, A.H.; Qureshi, M.S. Novel fork architectures of Ag2S nanoparticles synthesized through in-situ self-assembly inside chitosan matrix. Nano Hybrids 2012, 1, 23–43. [Google Scholar]
- Amah, A.N.; Echi, I.M.; Kalu, O. Influence of polyvinyl alcohol and alpha-methacrylic acid as capping agents on particle size of ZnS nanoparticles. Appl. Phys. Res. 2012, 4, 26–34. [Google Scholar]
- Liu, L.; Zheng, Z.; Wang, X. Preparation and properties of polythiourethane/ZnS nanocomposites with high refractive index. J. Appl. Polym. Sci. 2010, 117, 1978–1983. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mbese, J.Z.; Ajibade, P.A. Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites. Polymers 2014, 6, 2332-2344. https://doi.org/10.3390/polym6092332
Mbese JZ, Ajibade PA. Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites. Polymers. 2014; 6(9):2332-2344. https://doi.org/10.3390/polym6092332
Chicago/Turabian StyleMbese, Johannes Z., and Peter A. Ajibade. 2014. "Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites" Polymers 6, no. 9: 2332-2344. https://doi.org/10.3390/polym6092332
APA StyleMbese, J. Z., & Ajibade, P. A. (2014). Preparation and Characterization of ZnS, CdS and HgS/Poly(methyl methacrylate) Nanocomposites. Polymers, 6(9), 2332-2344. https://doi.org/10.3390/polym6092332