Preparation and Characterization of EG-Chitosan Nanocomposites via Direct Exfoliation: A Green Methodology
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
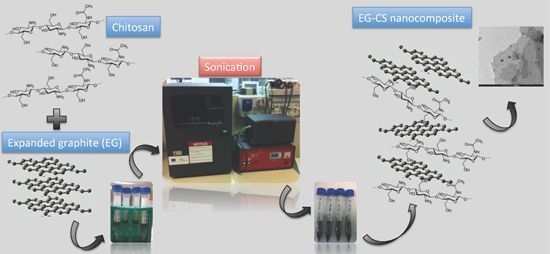
2.2. EG-Chitosan Nanocomposites Preparation
| Sample code | Chitosan solution (mL) | Sonication time (h) | EG (mg) |
|---|---|---|---|
| CS (control) | 3 | 0 | na |
| EG_CS 2h | 3 | 2 | 1.5 |
| EG_CS 4h | 3 | 4 | 1.5 |
| EG_CS 6h | 3 | 6 | 1.5 |
| EG_CS 8h | 3 | 8 | 1.5 |
2.3. Experimental Techniques
2.3.1. X-Ray Diffraction
2.3.2. TEM
2.3.3. DLS
2.3.4. UV-VIS
2.3.5. Thermal Analyses (TGA/DSC1)
3. Results and Discussion

| Sample code | 2θ (°) | d (nm) |
|---|---|---|
| GIC | 26.02; 26.55 | 0.336 |
| EG | 26.05; 26.56 | 0.335 |
| EG_CS 2h | 26.36 | 0.337 |
| EG_CS 8h | 26.28 | 0.338 |




| Sample code | Final solid residual (%) | EG weight fraction (%) |
|---|---|---|
| CS (control) | 0.02 | na |
| EG_CS 2h | 0.63 | 0.61 |
| EG_CS 4h | 0.89 | 0.87 |
| EG_CS 6h | 12.05 | 12.03 |
| EG_CS 8h | 24.64 | 24.68 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ruggerone, R.; Plummer, C.J.G.; Herrera, N.N.; Bourgeat-Lami, E.; Månson, J.A.E. Highly filled polystyrene-laponite nanocomposites prepared by emulsion polymerization. Eur. Polym. J. 2009, 45, 621–629. [Google Scholar] [CrossRef]
- Greco, A.; Corcione, C.E.; Strafella, A.; Maffezzoli, A. Analysis of the structure and mass transport properties of clay nanocomposites based on amorphous PET. J. Appl. Polym. Sci. 2010, 118, 3666–3672. [Google Scholar] [CrossRef]
- Ludueña, L.; Vázquez, A.; Alvarez, V. Viscoelastic behavior of polycaprolactone/clay nanocomposites. J. Compos. Mater. 2012, 46, 677–689. [Google Scholar] [CrossRef]
- Indennidate, L.; Cannoletta, D.; Lionetto, F.; Greco, A.; Maffezzoli, A. Nanofilled polyols for viscoelastic polyurethane foams. Polym. Int. 2010, 59, 486–491. [Google Scholar] [CrossRef]
- Lionetto, F.; Maffezzoli, A. Rheological characterization of concentrarted nanoclay dispersions in an organic solvent. Appl. Rheol. 2009, 19, 23423. [Google Scholar]
- Corcione, C.E.; Cavallo, A.; Pesce, E.; Greco, A.; Maffezzoli, A. Evaluation of the degree of dispersion of nanofillers by mechanical, rheological, and permeability analysis. Polym. Eng. Sci. 2011, 51, 1280–1285. [Google Scholar] [CrossRef]
- Rahatekar, S.S.; Zammarano, M.; Matko, S.; Koziol, K.K.; Windle, A.H.; Nyden, M.; Kashiwagi, T.; Gilman, J.W. Effect of carbon nanotubes and montmorillonite on the flammability of epoxy nanocomposites. Polym. Degrad. Stab. 2010, 95, 870–879. [Google Scholar] [CrossRef]
- Terenzi, A.; Vedova, C.; Lelli, G.; Mijovic, J.; Torre, L.; Valentini, L.; Kenny, J.M. Chemorheological behaviour of double-walled carbon nanotube-epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 1862–1868. [Google Scholar] [CrossRef]
- Pan, F.; Peng, F.; Jiang, Z. Diffusion behavior of benzene/cyclohexane molecules in poly(vinyl alcohol)-graphite hybrid membranes by molecular dynamics simulation. Chem. Eng. Sci. 2007, 62, 703–710. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet-epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Li, J.; Vaisman, L.; Marom, G.; Kim, J.K. Br treated graphite nanoplatelets for improved electrical conductivity of polymer composites. Carbon 2007, 45, 744–750. [Google Scholar] [CrossRef]
- Steiner, S.; Busato, S.; Ermanni, P. Mechanical properties and morphology of papers prepared from single-walled carbon nanotubes functionalized with aromatic amides. Carbon 2012, 50, 1713–1719. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Sun, X.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. Enhanced thermal conductivity in a hybrid graphite nanoplatelet—Carbon nanotube filler for epoxy composites. Adv. Mater. 2008, 20, 4740–4744. [Google Scholar] [CrossRef]
- Jović, N.; Dudić, D.; Montone, A.; Antisari, M.V.; Mitrić, M.; Djoković, V. Temperature dependence of the electrical conductivity of epoxy/expanded graphite nanosheet composites. Scr. Mater. 2008, 58, 846–849. [Google Scholar] [CrossRef]
- Veca, L.M.; Meziani, M.J.; Wang, W.; Wang, X.; Lu, F.; Zhang, P.; Lin, Y.; Fee, R.; Connell, J.W.; Sun, Y. Carbon nanosheets for polymeric nanocomposites with high thermal conductivity. Adv. Mater. 2009, 21, 2088–2092. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composite. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Zaman, I.; Phan, T.T.; Kuan, H.C.; Meng, Q.; La, L.T.B.; Luong, L.; Youssf, O.; Ma, J. Epoxy/graphene platelets nanocomposites with two levels of interface strength. Polymer 2011, 52, 1603–1611. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, C.; Song, L.; Yuan, B.; Hu, Y. In Situ polymerization of graphene, graphite oxide, and functionalized graphite oxide into epoxy resin and comparison study of on-the-flame behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Graphene–multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Dean, D.; Robinson, P.; Nyairo, E. Cure behavior of epoxy/MWCNT nanocomposites: The effect of nanotube surface modification. Polymer 2008, 49, 3310–3317. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Corcione, C.E.; Maffezzoli, A. Glass transition in thermosetting clay-nanocomposite polyurethanes. Thermochim. Acta 2009, 485, 43–48. [Google Scholar] [CrossRef]
- Corcione, C.E.; Freuli, F.; Maffezzoli, A. The aspect ratio of epoxy matrix nanocomposites reinforced with graphene stacks. Polym. Eng. Sci. 2013, 53, 531–539. [Google Scholar] [CrossRef]
- Choi, J.T.; Kim, D.H.; Ryu, K.S.; Lee, H.; Jeong, H.M.; Shin, C.M.; Kim, J.H.; Kim, B.K. Functionalized graphene sheet/polyurethane nanocomposites: Effect of particle size on physical properties. Macromol. Res. 2011, 19, 809–814. [Google Scholar] [CrossRef]
- Chan, C.M.; Wu, J.; Li, J.X.; Cheung, Y.K. Polypropylene/calcium carbonate nanocomposites. Polymer 2002, 43, 2981–2992. [Google Scholar] [CrossRef]
- Sumita, M.; Tsukumo, Y.; Miyasaka, K.; Ishikawa, K. Tensile yield stress of polypropylene composites filled with ultrafine particles. J. Mater. Sci. 1983, 18, 1758–1764. [Google Scholar] [CrossRef]
- Tien, Y.I.; Wei, K.H. High-tensile-property layered silicates/polyurethane nanocomposites by using reactive silicates as pseudo chain extenders. Macromolecules 2001, 34, 9045–9052. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: an overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Greco, A.; Maffezzoli, A.; Calò, E.; Massaro, C.; Terzi, R. An investigation into sintering of PA6 nanocomposite powders for rotational molding. J. Therm. Anal. Calorim. 2011, 109, 1493–1502. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D.; Wang, C. Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal. Chim. Acta 2012, 724, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Mu, L.; Wen, J.; Zhou, Q. Covalently synthesized graphene oxide-aptamer nanosheets for efficient visible-light photocatalysis of nucleic acids and proteins of viruses. Carbon 2012, 50, 2772–2781. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Shi, M.; Ma, H.; Li, N.; Ye, M. Synthesis of graphene oxide-based biocomposites through diimide-activated amidation. J. Colloid Interface Sci. 2011, 356, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.P.; Feng, H.F.; Xu, Z.Z.; Zhang, L.F.; Zhang, Y.L.; Xia, W.; Zhang, W.Q. Fabrication of biocompatible and mechanically reinforced graphene oxide-chitosan nanocomposite films. Chem. Cent. J. 2013, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Raucci, M.G.; Alvarez-Perez, M.A.; Demitri, C.; Giugliano, D.; de Benedictis, V.M.; Sannino, A.; Ambrosio, L. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. A 2015, 103, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Dimida, S.; Demitri, C.; de Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Madaghiele, M.; Sannino, A.; Ambrosio, L.; Demitri, C. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burn Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef]
- Demitri, C.; Giuri, A.; Raucci, M.G.; Giugliano, D.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Preparation and characterization of cellulose-based foams via microwave curing. Interface Focus 2014. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, T.; Bao, H.; Li, L. Green fabrication of chitosan films reinforced with parallel aligned graphene oxide. Carbohydr. Polym. 2011, 83, 1908–1915. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Datta, P.; Dhara, S.; Chatterjee, J. Hydrogels and electrospun nanofibrous scaffolds of N-methylene phosphonic chitosan as bioinspired osteoconductive materials for bone grafting. Carbohydr. Polym. 2012, 87, 1354–1362. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, Z.; Rouabhia, M.J. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. Bone Min. Metab. 2011, 29, 535–544. [Google Scholar]
- Corcione, C.E.; Maffezzoli, A. Transport properties of graphite/epoxy composites: Thermal, permeability and dielectric characterization. Polym. Test. 2013, 32, 880–888. [Google Scholar]
- Mauro, M.; Acocella, M.R.; Corcione, C.E.; Maffezzoli, A.; Guerra, G. Catalytic activity of graphite-based nanofillers on cure reaction of epoxy resins. Polymer 2014, 55, 5612–5615. [Google Scholar] [CrossRef]
- Chen, K.; Xue, D. Preparation of colloidal graphene in quantity by electrochemical exfoliation. J. Colloid Interface Sci. 2014, 436, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. Aip Adv. 2014, 4, 27116. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A general and efficient route to covalently surface modification of MWCNTs by dopamine and their synergistic reinforcing effects in chitosan films. Prog. Org. Coatings 2015, 85, 131–137. [Google Scholar] [CrossRef]
- Tian, H.; Tagaya, H. Preparation, characterization and mechanical properties of the polylactide/perlite and the polylactide/montmorillonite composites. J. Mater. Sci. 2007, 42, 3244–3250. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demitri, C.; Moscatello, A.; Giuri, A.; Raucci, M.G.; Esposito Corcione, C. Preparation and Characterization of EG-Chitosan Nanocomposites via Direct Exfoliation: A Green Methodology. Polymers 2015, 7, 2584-2594. https://doi.org/10.3390/polym7121535
Demitri C, Moscatello A, Giuri A, Raucci MG, Esposito Corcione C. Preparation and Characterization of EG-Chitosan Nanocomposites via Direct Exfoliation: A Green Methodology. Polymers. 2015; 7(12):2584-2594. https://doi.org/10.3390/polym7121535
Chicago/Turabian StyleDemitri, Christian, Anna Moscatello, Antonella Giuri, Maria Grazia Raucci, and Carola Esposito Corcione. 2015. "Preparation and Characterization of EG-Chitosan Nanocomposites via Direct Exfoliation: A Green Methodology" Polymers 7, no. 12: 2584-2594. https://doi.org/10.3390/polym7121535
APA StyleDemitri, C., Moscatello, A., Giuri, A., Raucci, M. G., & Esposito Corcione, C. (2015). Preparation and Characterization of EG-Chitosan Nanocomposites via Direct Exfoliation: A Green Methodology. Polymers, 7(12), 2584-2594. https://doi.org/10.3390/polym7121535