Active Peptide-Conjugated Chitosan Matrices as an Artificial Basement Membrane
Abstract
:1. Introduction

2. Cell-Adhesive Peptides from Laminin
| Peptide [Ref No.] | Sequence | Activity | Receptor |
|---|---|---|---|
| Chain (Residues) | |||
| A13 [33] | RQVFQVAYIIIKA | hepatocyte attachment | syndecan |
| mouse laminin α1 chain (121–133) | angiogenesis | integrin β1 | |
| A99 [53] | AGTFALRGDNPQG | cell spreading | integrin αvβ3 |
| mouse laminin α1 chain (1141–1153) | neurite outgrowth | ||
| metastasis suppression | |||
| A208 [65] | AASIKVAVSADR | fibril formation | 110-kDa protein |
| mouse laminin α1 chain (2121–2132) | neurite outgrowth | ||
| MMP↑ | |||
| AG73 [24] | RKRLQVQLSIRT | cell differentiation | syndecan |
| mouse laminin α1 chain (2719–2730) | neurite outgrowth | ||
| wound healing | |||
| EF-1 [38] | DYATLQLQEGRLHFMFDLG | cell spreading | integrin α2β1 |
| mouse laminin α1 chain (2747–2765) | |||
| C16 [35] | KAFDITYVRLKF | MMP↑ | unknown |
| mouse laminin γ1 chain (139–150) | angiogenesis | ||
| A2G10 [66] | SYWYRIEASRTG | cell spreading | integrin α6β1 |
| mouse laminin α2 chain (2223–2234) | |||
| A2G78 [ 29] | GLLFYMARINHA | SMG branching suppression | α-dystroglycan |
| mouse laminin α2 chain (2796–2807) | |||
| A2G80 [29] | VQLRNGFPYFSY | SMG branching suppression | α-dystroglycan |
| mouse laminin α2 chain (2812–2823) | |||
| A3G756 [22] | KNSFMALYLSKGRLVFALG | wound healing | syndecans |
| humanlaminin α3 chain (1411–1429) | |||
| A5G27 [67] | RLVSYNGIIFFLK | metastasis suppression | CD44 |
| mouse laminin α5 chain (2892–2904) |

3. Preparation of Peptide-Chitosan Matrices
4. Biological Functions of Peptide-Polysaccharide Complexes


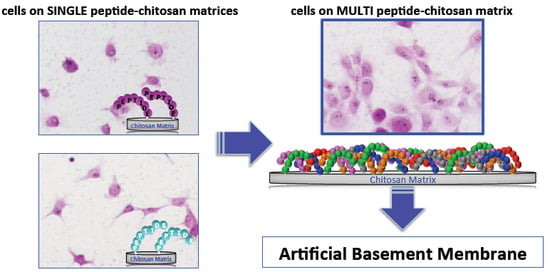
5. Mixed Peptide-Chitosan Matrices Mimic the Multifunctions of Laminins


| Group | Biological Activities on HDFs | Neurite Outgrowth Activity on PC12 cells | Peptides | ||||
|---|---|---|---|---|---|---|---|
| HDF Attachment | HDF Spreading | Inhibitory Effect on HDF Attachment | Predicted Cell Surface Receptors | ||||
| EDTA | Heparin | ||||||
| 1 | + | S | + | − | Integrin | + | A99a |
| 2 | + | S | + | − | Integrin | − | EF1zz |
| 3 | + | s | + | + | Integrin/syndecan | + | A13, AG32, AG103, C16, C57, C64 |
| 4 | + | s | + | + | Integrin/syndecan | − | A3, A55, A65, A119, A167, A174, AG10, AG28, AG56, B30, B133, B160, C59, C68 |
| 5 | + | w | − | + | Syndecan | + | A206, AG73, B20, B31 |
| 6 | − | − | − | − | − | + | A25, A112, A194 |

6. Application of Peptide-Chitosan Matrices to Cell Transplantation

7. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dieckmann, C.; Renner, R.; Milkova, L.; Simon, J.C. Regenerative medicine in dermatology: Biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp. Dermatol. 2010, 19, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 2014, 345. [Google Scholar] [CrossRef]
- Tuan, R.S. Regenerative medicine in 2012: The coming of age of musculoskeletal tissue engineering. Nat. Rev. Rheumatol. 2013, 9, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, J.; Miosge, N. Basement membrane components are key players in specialized extracellular matrices. Cell. Mol. Life Sci. 2010, 67, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Wiradjaja, F.; DiTommaso, T.; Smyth, I. Basement membranes in development and disease. Birth Defects Res. C Embryo Today Rev. 2010, 90, 8–31. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. J. Lab. Clin. Med. 2014, 163, 268–285. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.; Jorgensen, B.; Markowski, M.; Salchert, K.; Werner, C.; Pompe, T. Control of fibronectin displacement on polymer substrates to influence endothelial cell behaviour. J. Mater. Sci. Mater. Med. 2004, 15, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Patarroyo, M.; Tryggvason, K.; Virtanen, I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin. Cancer Biol. 2002, 12, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.H.; Yurchenco, P.D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2004, 20, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Scheele, S.; Nystrom, A.; Durbeej, M.; Talts, J.F.; Ekblom, M.; Ekblom, P. Laminin isoforms in development and disease. J. Mol. Med. 2007, 85, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Miyagoe, Y.; Hanaoka, K.; Nonaka, I.; Hayasaka, M.; Nabeshima, Y.; Arahata, K.; Nabeshima, Y.; Takeda, S. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 gene: A new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997, 415, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Thyboll, J.; Kortesmaa, J.; Cao, R.; Soininen, R.; Wang, L.; Iivanainen, A.; Sorokin, L.; Risling, M.; Cao, Y.; Tryggvason, K. Deletion of the laminin α4 chain leads to impaired microvessel maturation. Mol. Cell. Biol. 2002, 22, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Xu, H.; Vachon, P.H.; Engvall, E. Disruption of the Lama2 gene in embryonic stem cells: Laminin α2 is necessary for sustenance of mature muscle cells. Exp. Cell Res. 1998, 241, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.H.; Cunningham, J.; Sanes, J.R. Roles for laminin in embryogenesis: Exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J. Cell Biol. 1998, 143, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Lee, K.; Miyashita, Y.; Carter, W.G. Targeted disruption of the Lama3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J. Cell Biol. 1999, 145, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.H.; Li, C.; Mudd, J.L.; Go, G.; Sutherland, A.E. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 2004, 131, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hoshijima, M.; Lam, J.; Zhou, Z.; Jokiel, A.; Dalton, N.D.; Hultenby, K.; Ruiz-Lozano, P.; Ross, J., Jr.; Tryggvason, K.; et al. Cardiomyopathy associated with microcirculation dysfunction in laminin α4 chain-deficient mice. J. Biol. Chem. 2006, 281, 213–220. [Google Scholar]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Hozumi, K.; Katagiri, F.; Nomizu, M.; Kleinman, H.K.; Koblinski, J.E. Laminin-111-derived peptides and cancer. Cell Adhes. Migr. 2013, 7, 150–256. [Google Scholar] [CrossRef]
- Araki, E.; Momota, Y.; Togo, T.; Tanioka, M.; Hozumi, K.; Nomizu, M.; Miyachi, Y.; Utani, A. Clustering of syndecan-4 and integrin β1 by laminin α3 chain-derived peptide promotes keratinocyte migration. Mol. Biol. Cell 2009, 20, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Chen, L.; Woods, A. Syndecans and cell adhesion. Int. Rev. Cytol. 2001, 207, 113–150. [Google Scholar] [PubMed]
- Hoffman, M.P.; Nomizu, M.; Roque, E.; Lee, S.; Jung, D.W.; Yamada, Y.; Kleinman, H.K. Laminin-1 and laminin-2 g-domain synthetic peptides bind syndecan-1 and are involved in acinar formation of a human submandibular gland cell line. J. Biol. Chem. 1998, 273, 28633–28641. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Suzuki, N.; Nielsen, P.K.; Nomizu, M.; Yamada, Y. Laminin α1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin α2β1. J. Biol. Chem. 2006, 281, 32929–32940. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Couchman, J.R. Syndecans: Synergistic activators of cell adhesion. Trends Cell Biol. 1998, 8, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Miner, J.H. Review: Lutheran/B-CAM: A laminin receptor on red blood cells and in various tissues. Connect. Tissue Res. 2005, 46, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M.; Henry, M.D.; Campbell, K.P. Dystroglycan in development and disease. Curr. Opin. Cell Biol. 1998, 10, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hozumi, K.; Urushibata, S.; Yoshimura, T.; Kikkawa, Y.; Gumerson, J.D.; Michele, D.E.; Hoffman, M.P.; Yamada, Y.; Nomizu, M. Identification of α-dystroglycan binding sequences in the laminin α2 chain LG4–5 module. Matrix Biol. J. Int. Soc. Matrix Biol. 2010, 29, 143–151. [Google Scholar] [CrossRef]
- Charonis, A.; Sideraki, V.; Kaltezioti, V.; Alberti, A.; Vlahakos, D.; Wu, K.; Tsilibary, E. Basement membrane peptides: Functional considerations and biomedical applications in autoimmunity. Curr. Med. Chem. 2005, 12, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Akizuki, T.; Yamada, Y.; Hara, T.; Urushibata, S.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Cell adhesive peptide screening of the mouse laminin α1 chain G domain. Arch. Biochem. Biophys. 2010, 503, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Nomizu, M.; Kim, W.H.; Yamamura, K.; Utani, A.; Song, S.Y.; Otaka, A.; Roller, P.P.; Kleinman, H.K.; Yamada, Y. Identification of cell binding sites in the laminin α1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J. Biol. Chem. 1995, 270, 20583–20590. [Google Scholar] [CrossRef] [PubMed]
- Nomizu, M.; Kuratomi, Y.; Malinda, K.M.; Song, S.Y.; Miyoshi, K.; Otaka, A.; Powell, S.K.; Hoffman, M.P.; Kleinman, H.K.; Yamada, Y. Cell binding sequences in mouse laminin α1 chain. J. Biol. Chem. 1998, 273, 32491–32499. [Google Scholar] [CrossRef] [PubMed]
- Nomizu, M.; Kuratomi, Y.; Ponce, M.L.; Song, S.Y.; Miyoshi, K.; Otaka, A.; Powell, S.K.; Hoffman, M.P.; Kleinman, H.K.; Yamada, Y. Cell adhesive sequences in mouse laminin β1 chain. Arch. Biochem. Biophys. 2000, 378, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Nomizu, M.; Kuratomi, Y.; Song, S.Y.; Ponce, M.L.; Hoffman, M.P.; Powell, S.K.; Miyoshi, K.; Otaka, A.; Kleinman, H.K.; Yamada, Y. Identification of cell binding sequences in mouse laminin γ1 chain by systematic peptide screening. J. Biol. Chem. 1997, 272, 32198–32205. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yokoyama, F.; Nomizu, M. Functional sites in the laminin alpha chains. Connect. Tissue Res. 2005, 46, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Nomizu, M.; Weeks, B.S.; Weston, C.A.; Kim, W.H.; Kleinman, H.K.; Yamada, Y. Structure-activity study of a laminin α1 chain active peptide segment Ile-Lys-Val-Ala-Val (IKVAV). FEBS Lett. 1995, 365, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Nakatsuka, H.; Mochizuki, M.; Nishi, N.; Kadoya, Y.; Utani, A.; Oishi, S.; Fujii, N.; Kleinman, H.K.; Nomizu, M. Biological activities of homologous loop regions in the laminin α chain G domains. J. Biol. Chem. 2003, 278, 45697–45705. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Minami, S. Applications of chitin and chitosan for biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 383–420. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Liu, X.; Ma, L.; Mao, Z.; Gao, C. Chitosan-based biomaterials for tissue repair and regeneration. Adv. Polym. Sci. 2011, 244, 81–128. [Google Scholar]
- Chen, H.; Liu, Y.; Jiang, Z.; Chen, W.; Yu, Y.; Hu, Q. Cell-scaffold interaction within engineered tissue. Exp. Cell Res. 2014, 323, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chung, Y.C.; Wang, I.J.; Young, T.H. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials 2012, 33, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lu, X.; Hong, Y.; Xi, T.; Zhang, D. The molecular mechanism of mediation of adsorbed serum proteins to endothelial cells adhesion and growth on biomaterials. Biomaterials 2013, 34, 5747–5758. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, N.; Ravichandran, P.; Reddy, P.N.; Ramamurty, N.; Pal, S.; Rao, K.P. Collagen-chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials 2001, 22, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, M.G.; Guan, Y.J.; Schreyer, D.J.; Chen, X.B. Effects of laminin blended with chitosan on axon guidance on patterned substrates. Biofabrication 2010, 2. [Google Scholar] [CrossRef]
- Martin-Lopez, E.; Alonso, F.R.; Nieto-Diaz, M.; Nieto-Sampedro, M. Chitosan, gelatin and poly(l-lysine) polyelectrolyte-based scaffolds and films for neural tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 207–232. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Iwasaki, N.; Yamane, S.; Funakoshi, T.; Majima, T.; Minami, A.; Ohsuga, N.; Ohta, T.; Nishimura, S. Chitosan-rgdsggc conjugate as a scaffold material for musculoskeletal tissue engineering. Biomaterials 2005, 26, 5339–5347. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Kadoya, Y.; Wakabayashi, Y.; Kato, K.; Okazaki, I.; Yamada, M.; Sato, T.; Sakairi, N.; Nishi, N.; Nomizu, M. Laminin-1 peptide-conjugated chitosan membranes as a novel approach for cell engineering. FASEB J. 2003, 17, 875–877. [Google Scholar] [PubMed]
- Hozumi, K.; Otagiri, D.; Yamada, Y.; Sasaki, A.; Fujimori, C.; Wakai, Y.; Uchida, T.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Cell surface receptor-specific scaffold requirements for adhesion to laminin-derived peptide-chitosan membranes. Biomaterials 2010, 31, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hozumi, K.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Laminin-111-derived peptide-hyaluronate hydrogels as a synthetic basement membrane. Biomaterials 2013, 34, 6539–6547. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hozumi, K.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Biological activity of laminin peptide-conjugated alginate and chitosan matrices. Biopolymers 2010, 94, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hozumi, K.; Nomizu, M. Construction and activity of a synthetic basement membrane with active laminin peptides and polysaccharides. Chemistry 2011, 17, 10500–10508. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.; Hashom, N.; Falson, F.; Rousselle, P.; Jordan, O.; Borchard, G. In vitro evaluation of an rgd-functionalized chitosan derivative for enhanced cell adhesion. Carbohydr. Polym. 2012, 90, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Kobayashi, H.; Itoh, S.; Kataoka, K.; Tanaka, J. Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding. Biomaterials 2005, 26, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Sasaki, A.; Yamada, Y.; Otagiri, D.; Kobayashi, K.; Fujimori, C.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Reconstitution of laminin-111 biological activity using multiple peptide coupled to chitosan scaffolds. Biomaterials 2012, 33, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Yamagata, N.; Otagiri, D.; Fujimori, C.; Kikkawa, Y.; Kadoya, Y.; Nomizu, M. Mixed peptide-chitosan membranes to mimic the biological activities of a multifunctional laminin α1 chain LG4 module. Biomaterials 2009, 30, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S.; Mochizuki, M.; Yamada, M.; Takeda, A.; Uchinuma, E.; Yamashina, S.; Nomizu, M.; Kadoya, Y. Laminin peptide-conjugated chitosan membrane: Application for keratinocyte delivery in wounded skin. J. Biomed. Mater. Res. A 2006, 79, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamagata, N.; Philp, D.; Hozumi, K.; Watanabe, T.; Kikkawa, Y.; Kadoya, Y.; Kleinman, H.K.; Nomizu, M. Integrin-dependent cell behavior on ECM peptide-conjugated chitosan membranes. Biopolymers 2007, 88, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Otagiri, D.; Yamada, Y.; Hozumi, K.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Cell attachment and spreading activity of mixed laminin peptide-chitosan membranes. Biopolymers 2013, 100, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Urushibata, S.; Hozumi, K.; Yokoyama, F.; Ichikawa, N.; Kadoya, Y.; Nishi, N.; Watanabe, N.; Yamada, Y.; Nomizu, M. Identification of multiple amyloidogenic sequences in laminin-1. Biochemistry 2007, 46, 3966–3974. [Google Scholar] [CrossRef] [PubMed]
- Urushibata, S.; Hozumi, K.; Ishikawa, M.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Identification of biologically active sequences in the laminin α2 chain G domain. Arch. Biochem. Biophys. 2010, 497, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Shibuya, M.; Hoffman, M.P.; Engbring, J.A.; Hossain, R.; Mochizuki, M.; Kudoh, S.; Nomizu, M.; Kleinman, H.K. Laminin α5 chain metastasis- and angiogenesis-inhibiting peptide blocks fibroblast growth factor 2 activity by binding to the heparan sulfate chains of CD44. Cancer Res. 2005, 65, 10494–10501. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Philp, D.; Hozumi, K.; Suzuki, N.; Yamada, Y.; Kleinman, H.K.; Nomizu, M. Angiogenic activity of syndecan-binding laminin peptide AG73 (RKRLQVQLSIRT). Arch. Biochem. Biophys. 2007, 459, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Shibuya, M.; Engbring, J.A.; Mochizuki, M.; Nomizu, M.; Kleinman, H.K. Identification of an active site on the laminin α5 chain globular domain that binds to CD44 and inhibits malignancy. Cancer Res. 2004, 64, 4810–4816. [Google Scholar] [CrossRef] [PubMed]
- Streuli, C.H.; Akhtar, N. Signal co-operation between integrins and other receptor systems. Biochem. J. 2009, 418, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Masuda, R.; Mochizuki, M.; Hozumi, K.; Takeda, A.; Uchinuma, E.; Yamashina, S.; Nomizu, M.; Kadoya, Y. A novel cell-adhesive scaffold material for delivering keratinocytes reduces granulation tissue in dermal wounds. Wound Repair Regen. 2009, 17, 127–135. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hozumi, K.; Kumai, J.; Yamada, Y.; Nomizu, M. Active Peptide-Conjugated Chitosan Matrices as an Artificial Basement Membrane. Polymers 2015, 7, 281-297. https://doi.org/10.3390/polym7020281
Hozumi K, Kumai J, Yamada Y, Nomizu M. Active Peptide-Conjugated Chitosan Matrices as an Artificial Basement Membrane. Polymers. 2015; 7(2):281-297. https://doi.org/10.3390/polym7020281
Chicago/Turabian StyleHozumi, Kentaro, Jun Kumai, Yuji Yamada, and Motoyoshi Nomizu. 2015. "Active Peptide-Conjugated Chitosan Matrices as an Artificial Basement Membrane" Polymers 7, no. 2: 281-297. https://doi.org/10.3390/polym7020281
APA StyleHozumi, K., Kumai, J., Yamada, Y., & Nomizu, M. (2015). Active Peptide-Conjugated Chitosan Matrices as an Artificial Basement Membrane. Polymers, 7(2), 281-297. https://doi.org/10.3390/polym7020281