Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications
Abstract
:1. Introduction

2. Experimental Section
3. MC Synthesis and Characterization
3.1. Industrial Preparation/Homogeneous Synthesis
3.2. Characterization of MC
3.2.1. Substitution Pattern


| DS | A15LV | A4C | A15C | A4M |
|---|---|---|---|---|
| DS2+3 | 0.82 | 0.82 | 0.84 | 0.90 |
| DS6 | 0.47 | 0.47 | 0.51 | 0.56 |
| Samples | [η] a (mL·g−1) | C* (g·L−1) | MV b (g·mol−1) | DS c | DS2 | DS3 | DS6 |
|---|---|---|---|---|---|---|---|
| A15LV | 193 | 5.18 | 42,100 | 1.8 | 0.8 | 0.4 | 0.6 |
| A4C | 573 | 1.75 | 212,000 | 1.7 | 0.7 | 0.5 | 0.5 |
| A15C | 740 | 1.35 | 304,600 | 1.8 | 0.7 | 0.5 | 0.6 |
| A4M | 933 | 1.07 | 423,400 | 1.7 | 0.7 | 0.5 | 0.5 |
| Data | A15LV | A4C | A15C | A4M |
|---|---|---|---|---|
| DS a | 1.74 | 1.73 | 1.71 | 1.73 |
| % NoS b | 12 | 10 | 10 | 11 |
| % MonoS c | 26 | 29 | 27 | 27 |
| % DiS d | 38 | 39 | 39 | 40 |
| % TriS e | 24 | 22 | 24 | 22 |
3.2.2. Macromolecular Characterization
4. Physical Properties in Aqueous Solution
4.1. Solubility in Relation to Temperature

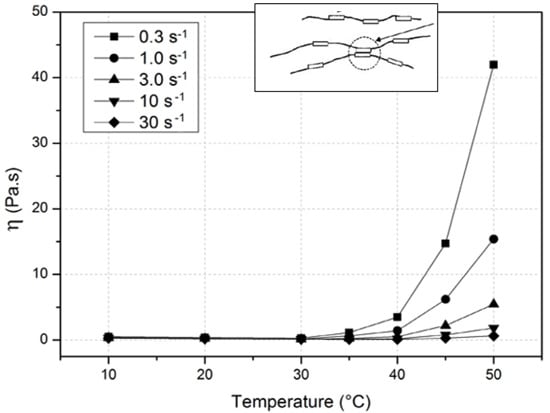
4.2. Rheological Behavior Up to Gelation

| Critical Temperature | A15LV | A4C | A15C | A4M |
|---|---|---|---|---|
| Tc (°C) | 40 | 40 | 35 | 30 |


4.3. Influence of the Molecular Weight and Concentration on the Mechanism of Gelation



- Firstly, at relatively low temperatures and low concentrations, a sol–gel domain corresponds to association of the most hydrophobic zones of few chains. The chain heterogeneity is caused by the highly methylated blocks.
- Secondly, at higher temperatures, a concomitant gel is formed corresponding to microphase separation in which polymer-rich microdomains prevent phase separation leading to a turbid gel. The mobility of the chains is reduced when the phase separation process is slow. This turbid gel is metastable and finally collapses with exclusion of solvent (syneresis process) especially at high temperature (>70 °C) and low concentration as also found on κ-carrageenan [4,10,13,67].
4.4. Gel Structure and Hysteresis
4.5. Interfacial Properties
4.5.1. Surface Activity of Methylcelluloses

4.5.2. Adsorption Isotherm

5. Applications
5.1. Main Properties
5.2. Main Applications
5.2.1. Food
5.2.2. Cosmetics and Personal Care
5.2.3. Pharmaceutical and Biomedical
5.2.4. Ceramics and Construction Materials
5.2.5. Adhesives
5.2.6. Agriculture
5.2.7. Other Applications
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coffey, D.G.; Bell, D.A.; Henderson, A. Cellulose and cellulose derivatives. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., William, P.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1995; Volume 5, pp. 123–153. [Google Scholar]
- Methocel Cellulose Ethers. Technical Handbook. Available online: www.dow.com/dowwolff/en/pdf/192-01062.pdf (accessed on 20 April 2015).
- Methylcellulose, Methylhydroxypropylcellulose, Hypromellose. Physical and Chemical Properties. Available online: http://www.brenntagspecialties.com/en/downloads/Products/Multi_Market_Principals/Aqualon/Benecel_Brochure.pdf (accessed on 20 April 2015).
- Chevillard, C.; Axelos, M.A.V. Phase separation of aqueous solution of methylcellulose. Colloid Polym. Sci. 1997, 275, 537–545. [Google Scholar] [CrossRef]
- Ruta, B.; Czakkel, O.; Chusshkin, Y.; Pignon, F.; Zontone, F.; Rinaudo, M. Silica nanoparticles as tracers of the gelation dynamics of a natural biopolymer physical gel. Soft Matter 2014, 10, 4547–4554. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, S.A.; Lott, J.R.; McAllister, J.W.; Zhang, J.; Bates, F.S.; Lodge, T.P.; Sammler, R.L.; Li, Y.; Brackhagen, M. Interplay of phase separation and thermoreversible gelation in aqueous methylcellulose solutions. Macromolecules 2013, 46, 300–309. [Google Scholar] [CrossRef]
- Takahashi, M.; Shimazaki, M.; Yamamoto, J. Thermoreversible gelation and phase separation in aqueous methyl cellulose solutions. J. Polym. Sci. B Polym. Phys. 2001, 39, 91–100. [Google Scholar] [CrossRef]
- Haque, A.; Morris, E.R. Thermogelation of methylcellulose. Part I: Molecular structures and processes. Carbohydr. Polym. 1993, 22, 161–173. [Google Scholar] [CrossRef]
- Funami, T.; Kataoka, Y.; Hiroe, M.; Asai, I.; Takahashi, R.; Nishinari, K. Thermal aggregation of methylcellulose with different molecular weights. Food Hydrocoll. 2007, 21, 46–58. [Google Scholar] [CrossRef]
- Hirrien, M.; Chevillard, C.; Descrières, J.; Axelos, M.A.V.; Rinaudo, M. Thermogelation of methylcelluloses: New evidence for understanding the gelation mechanism. Polymer 1998, 39, 6251–6259. [Google Scholar] [CrossRef]
- Vigouret, M.; Rinaudo, M.; Desbrières, J. Thermogelation of methylcellulose in aqueous solutions. J. Chim. Phys. 1996, 93, 858–869. [Google Scholar]
- Hirrien, M.; Desbrières, J.; Rinaudo, M. Physical properties of methylcelluloses in relation with the conditions for cellulose modification. Carbohydr. Polym. 1996, 31, 243–253. [Google Scholar] [CrossRef]
- Kato, T.; Yokoyama, M.; Takahashi, A. Melting temperatures of thermally reversible gels. Colloid Polym. Sci. 1978, 256, 15–21. [Google Scholar] [CrossRef]
- Sarkar, N. Kinetics of thermal gelation of methylcellulose and hydroxypropylmethylcellulose in aqueous solutions. Carbohydr. Polym. 1995, 26, 195–203. [Google Scholar] [CrossRef]
- Li, L.; Thangamathesvaran, P.M.; Yue, C.Y.; Tam, K.C.; Hu, X.; Lam, Y.C. Gel network structure of methylcellulose in water. Langmuir 2001, 17, 8062–8068. [Google Scholar] [CrossRef]
- Bain, M.K.; Bhowmick, B.; Maity, D.; Mondal, D.; Mollick, M.M.R.; Rana, D.; Chattopadhyay, D. Synergstic effect of salt mixture on the gelation temperature and morphology of methylcellulose hydrogel. Int. J. Biol. Macromol. 2012, 51, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Hirrien, M. Comportement des Méthylcelluloses en Relation Avec leur Structure. Ph.D. Thesis, Joseph Fourier University, Grenoble, France, December 1996. [Google Scholar]
- Wüstenberg, T.E.D. Fundamentals of water-soluble cellulose ethers and methylcelluloses. In Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2014; Chapter 5; pp. 185–274. [Google Scholar]
- Nishinari, K. Rheological and DSC study of sol–gel transition in aqueous dispersion of industrially important polymers and colloids. Colloid Polym. Sci. 1997, 275, 1093–1107. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Influence of molar mass and concentration on the thermogelation of methylcelluloses. Int. J. Polym. Anal. Charact. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Bodvik, R.; Karlson, L.; Edwards, K.; Eriksson, J.; Thormann, E.; Claesson, P.M. Aggregation of modified celluloses in aqueous solution: Transition from methylcellulose to hydroxypropylmethylcellulose solution properties induced by a low-molecular-weight oxyethylene additive. Langmuir 2012, 28, 13562–13569. [Google Scholar] [CrossRef] [PubMed]
- Lott, J.R.; McAllister, J.W.; Wasbrough, M.; Sammler, R.L.; Bates, F.S.; Lodge, T.P. Fibrillar structure in aqueous methylcellulose solutions and gels. Macromolecules 2013, 46, 9760–9771. [Google Scholar] [CrossRef]
- Lott, J.R.; McAllister, J.W.; Arvidson, S.A.; Bates, F.S.; Lodge, T.P. Fibrillar structure of methylcellulose hydrogels. Biomacromolecules 2013, 14, 2484–2488. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Interfacial properties of methylcelluloses: The Influence of molar mass. Polymers 2014, 6, 2961–2973. [Google Scholar] [CrossRef]
- Ibbett, R.N.; Philp, K.; Price, D.M. 13C NMR, studies of the thermal behavior of aqueous solutions of cellulose ethers. Polymer 1992, 33, 4087–4094. [Google Scholar] [CrossRef]
- Sachinvala, N.D.; Hamed, O.A.; Winsor, D.L.; Neimczura, W.P.; Maskos, K.; Parikh, D.V.; Glasser, W.; Becker, U.; Blanchard, E.J.; Bertoniere, N.R.; et al. Characterization of tri-O-methylcellulose by one and two-dimensional NMR methods. J. Polym. Sci. A Polym. Chem. 1999, 37, 4019–4032. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Sawatari, C.; Kondo, T. A facile method of determination for distribution of the substituent in O-methylcelluloses using 1H-NMR spectroscopy. Polym. Bull. 2002, 47, 547–554. [Google Scholar] [CrossRef]
- Karakawa, M.; Mikawa, Y.; Kamitakahara, H.; Nakatsubo, F. Preparations of regioselectively methylated cellulose acetates and their 1H and 13C NMR spectroscopic analyses. J. Polym. Sci. A Polym. Chem. 2002, 40, 4167–4179. [Google Scholar] [CrossRef]
- Takahashi, S.; Fujimoto, T.; Miyamoto, T.; Inagaki, H. Relationship between distribution of substituents and water solubility of O-methyl cellulose. J. Polym. Sci. A. Polym. Chem. 1987, 25, 987–994. [Google Scholar] [CrossRef]
- Liu, H.-Q.; Zhang, L.; Takaragi, A.; Miyamoto, T. Phase transition of 2,3-O-methylcellulose. Polym. Bull. 1998, 40, 741–747. [Google Scholar] [CrossRef]
- Nishinari, K.; Hofmann, K.E.; Moritaka, H.; Kohyama, K.; Nishinari, N. Gel–sol transition of methylcellulose. Macromol. Chem. Phys. 1997, 198, 1217–1226. [Google Scholar] [CrossRef]
- Klemm, D.; Phillip, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry: Fundamentals and Analytical Methods; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2004; Volume 1. [Google Scholar]
- Mansour, O.Y.; Nagaty, A.; El-Zawawy, W.K. Variables affecting the methylation reactions of cellulose. J. Appl. Polym. Sci. 1994, 54, 519–524. [Google Scholar] [CrossRef]
- Arisz, P.W.; Kauw, H.J.J.; Boon, J.J. Substituent distribution along the cellulose backbone in O-methylcelluloses using GC and FAB-MS for monomer and oligomer analysis. Carbohydr. Res. 1995, 271, 1–14. [Google Scholar] [CrossRef]
- Klemm, D.; Heinze, T.; Philipp, B.; Wagenknecht, W. New approaches to advanced polymers by selective cellulose functionalization. Acta Polym. 1997, 48, 277–297. [Google Scholar] [CrossRef]
- Heinze, T. Chemical functionalization of cellulose. In Polysaccharides: Structural Diversity and Functional Versability, 2nd ed.; Dumitru, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; Chapter 23; pp. 551–590. [Google Scholar]
- Ye, D.; Farriol, X. Improving accessibility and reactivity of celluloses of annual plants for the synthesis of methylcellulose. Cellulose 2005, 12, 507–515. [Google Scholar] [CrossRef]
- Viera, R.G.P.; Filho, G.R.; Assunção, R.M.N.; Meireles, C.S.; Vieira, J.G.; Oliveira, G.S. Synthesis and characterization of methylcelluloses from sugar cane bagasse cellulose. Carbohydr. Polym. 2007, 67, 182–189. [Google Scholar] [CrossRef]
- Oliveira, G.C.; Filho, G.R.; Vieira, J.G.; Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Oliveira, R.J.; Silva, W.G.; Motta, L.A.C. Synthesis and application of methylcellulose extracted from waste newspaper in CPV-ARI Portland Cement Mortars. J. Appl. Polym. Sci. 2010, 118, 1380–1385. [Google Scholar]
- Bock, L.H. Water-soluble cellulose ethers—A new method of preparation and theory of solubility. Ind. Eng. Chem. 1937, 29, 985–987. [Google Scholar] [CrossRef]
- McCormick, C.L.; Shen, T.S. Cellulose dissolution and derivatization in lithium chloride/N,N-dimethylacetamide solutions. In Macromolecular Solutions. Solvent-Property Relationships in Polymers; Seymour, R.B., Stahl, G.A., Eds.; Elsevier BV: New-York, NY, USA, 1982; pp. 101–107. [Google Scholar]
- Ke, H.; Zhou, J.; Zhang, L.; Zhang, L. Structure and physical properties of methylcellulose synthesized in NaOH/urea solution. Polym. Bull. 2006, 56, 349–357. [Google Scholar] [CrossRef]
- Kern, H.; Choi, S.W.; Wens, G.; Heinrich, J.; Ehrhardt, L.; Mischnick, P.; Garidel, P.; Blume, A. Synthesis, control of substitution pattern and phase transition of 2,3-di-O-methylcellulose. Carbohydr. Res. 2000, 326, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Koschella, A.; Heublein, B.; Klemm, D.; Heinze, T. Hydrogen bond formation in regioselecticely functionalized 3-mono-O-methyl cellulose. Carbohydr. Res. 2008, 343, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, H.; Koschella, A.; Mikawa, Y.; Nakatsubo, F.; Heinze, T.; Klemm, D. Syntheses and comparison of 2,6-Di-O-methyl celluloses from natural and synthetic celluloses. Macromol. Biosci. 2008, 8, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Hearon, W.M.; Hiatt, G.D.; Fordyce, C.R. Cellulose trityl ether1a. J. Am. Chem. Soc. 1943, 65, 2449–2452. [Google Scholar] [CrossRef]
- Green, J.W. Triphenylmethyl ethers. In Methods in Carbohydrate Chemistry, Volume III: Cellulose; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1963; p. 327. [Google Scholar]
- Camacho Gomez, J.A.; Erler, U.W.; Klemm, D. 4-Methoxy substituted trityl groups in 6-O protection of cellulose: Homogeneous synthesis, characterization, detritylation. Macromol. Chem. Phys. 1996, 197, 953–964. [Google Scholar] [CrossRef]
- Sarkar, N. Structural interpretation of the interfacial properties of aqueous solutions of methylcellulose and hydroxypropyl methylcellulose. Polymer 1984, 25, 481–486. [Google Scholar] [CrossRef]
- Bayer, R.; Knarr, M. Thermal precipitation or gelling behavior of dissolved methylcellulose (MC) derivatives—Behaviour in water and influence on the extrusion of ceramic pastes. Part 1: Fundamentals of MC-derivatives. J. Eur. Ceram. Soc. 2012, 32, 1007–1018. [Google Scholar] [CrossRef]
- Erler, U.; Mischnick, P.; Stein, A.; Klemm, D. Determination of the substitution patterns of cellulose methyl ethers by HPLC and GLC—Comparison of methods. Polym. Bull. 1992, 29, 349–356. [Google Scholar] [CrossRef]
- Bodvik, R.; Dedinaite, A.; Karlson, L.; Bergström, M.; Bäverbäck, P.; Pedersen, J.S.; Edwards, K.; Karlsson, G.; Varga, I.; Claesson, P.M.; et al. Aggregation and network formation of aqueous methylcellulose and hydroxypropulmethylcellulose solutions. Colloid Surf. A 2010, 354, 162–171. [Google Scholar] [CrossRef]
- Chatterjee, T.; Nakatani, A.I.; Adden, R.; Brackhagen, M.; Redwine, D.; Shen, H.; Li, Y.; Wilson, T.; Sammler, R.L. Structure and properties of aqueous methylcellulose gels by small-angle neutron scattering. Biomacromolecules 2012, 13, 3355–3369. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Huang, C.; Lodge, T.P. Thermoreversible gelation of aqueous methylcellulose solutions. Macromolecules 1999, 32, 7070–7077. [Google Scholar] [CrossRef]
- Uda, V.K.; Meyerhoff, G. Hydrodynamische eigenschaften von methylcellulosen in lösung. Makromol. Chem. 1961, 47, 168–184. (In German) [Google Scholar] [CrossRef]
- Keary, C.M. Characterization of METHOCEL cellulose ethers by aqueous SEC with multiple detectors. Carbohydr. Polym. 2001, 45, 293–303. [Google Scholar] [CrossRef]
- Sarkar, N.; Cutié, S. Private Communication, 1993.
- Poché, D.S.; Ribes, A.J.; Tipton, D.L. Characterization of cellulose ether: Correlation of static light-scattering data to GPC molar mass data based on pullulan standards. J. Appl. Polym. Sci. 1998, 70, 2197–2210. [Google Scholar] [CrossRef]
- Cox, W.P.; Merz, E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Knarr, M.; Bayer, R. The shear dependence of the methylcellulose gelation phenomena in aqueous solution and in ceramic paste. Carbohydr. Polym. 2014, 111, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Desbrières, J.; Hirrien, M.; Ross-Murphy, S.B. Thermogelation of methylcellulose: Rheological considerations. Polymers 2000, 41, 2451–2461. [Google Scholar] [CrossRef]
- Fairclough, J.P.A.; Yu, H.; Kelly, O.; Ryan, A.J.; Sammler, R.L.; Radler, M. Interplay between gelation and phase separation in aqueous solutions of mehtylcellulose and hydroxypropylmethylcellulose. Langmuir 2012, 28, 10551–10557. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Moroni, A. Rheological behavior of binary and ternary mixtures of polysaccharides in aqueous medium. Food Hydrocoll. 2009, 23, 1720–1728. [Google Scholar] [CrossRef]
- Takeshita, H.; Saito, K.; Miya, M.; Takenaka, K.; Shiomi, T. Laser speckle analysis on correlation between gelation and phase separation in aqueous methyl cellulose solutions. J. Polym. Sci. B Polym. Phys. 2010, 48, 168–174. [Google Scholar] [CrossRef]
- Rinaudo, M. Gelation of polysaccharides. J. Intell. Mater. Syst. Struct. 1993, 4, 210–215. [Google Scholar] [CrossRef]
- Milas, M.; Rinaudo, M. The gellan sol–gel transition. Carbohydr. Polym. 1996, 30, 177–184. [Google Scholar] [CrossRef]
- Landry, S. Relation Entre la Structure Moléculaire et les Propriétés Mécaniques des Gels de Carraghénanes. Ph.D. Thesis, Joseph Fourier University, Grenoble, France, December 1987. [Google Scholar]
- Kundu, P.P.; Kundu, M.; Sinha, M.; Choe, S.; Chattopadhayay, D. Effect of alcoholic, glycolic, and polyester resin additives on the gelation of dilute solution (1%) of methylcellulose. Carbohydr. Polym. 2003, 51, 57–61. [Google Scholar] [CrossRef]
- Dickinson, E. Milk protein interfacial layers and the relationship to emulsion stability and rheology. Colloid Surf. B 2001, 20, 197–210. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Rinaudo, M.; Quemeneur, F.; Pepin-Donat, B. Stabilization of liposomes by polyelectrolytes: Mechanism of interaction and role of experimental conditions. Macromol. Symp. 2009, 278, 67–79. [Google Scholar] [CrossRef]
- Quemeneur, F.; Rinaudo, M.; Maret, G.; Pepin-Donat, B. Decoration of lipid vesicles by polyelectrolytes: Mechanism and structure. Soft Matter 2010, 6, 4471–4481. [Google Scholar] [CrossRef]
- Daniels, R.; Barta, A. Pharmacopeial cellulose ethers as oil-in-water emulsifiers 1. Interfacial properties. Eur. J. Pharm. Biopharm. 1994, 40, 128–133. [Google Scholar]
- Sarker, D.K.; Axelos, M.; Popineau, Y. Methylcellulose-induced stability changes in protein-based emulsions. Colloid Surf. B. 1999, 12, 147–160. [Google Scholar] [CrossRef]
- Gaonkar, A.G. Surface and interfacial activities and emulsion characteristics of some food hydrocolloids. Food Hydrocoll. 1991, 5, 329–337. [Google Scholar] [CrossRef]
- Arboleya, J.-C.; Wilde, P.J. Competitive adsorption of proteins with methylcellulose and hydroxypropylmethylcellulose. Food Hydrocoll. 2005, 19, 485–491. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goff, H.D.; Smith, A.K. Texture–structure relationships in foamed dairy Emulsions. Food Res. Int. 1996, 29, 1–13. [Google Scholar] [CrossRef]
- Babak, V.G.; Auzely, R.; Rinaudo, M. Effect of electrolyte concentration on the dynamic surface tension and dilational viscoelasticity of adsorption layers of chitosan and dodecyl chitosan. J. Phys. Chem. B 2007, 111, 9519–9529. [Google Scholar] [CrossRef] [PubMed]
- Desbrieres, J.; Rinaudo, M.; Babak, V.; Vikhoreva, G. Surface activity of water soluble amphiphilic chitin derivatives. Polym. Bull. 1997, 39, 209–215. [Google Scholar] [CrossRef]
- Babak, V.; Lukina, I.; Vikhoreva, G.; Desbrieres, J.; Rinaudo, M. Interfacial properties of dynamic association between chitin derivatives and surfactants. Colloid Surf. A 1999, 147, 139–148. [Google Scholar] [CrossRef]
- Nahringbauer, I. Dynamic surface tension of aqueous polymer solutions, I. Ethyl(hydroxyethyl) cellulose (BERMOCOLL cst-103). J. Colloid Interface Sci. 1995, 112, 318–328. [Google Scholar] [CrossRef]
- Park, Y.; Huang, R.; Corti, D.S.; Franses, E.J. Colloidal dispersion stability of unilamellar DPPC vesicle in aqueous electrolyte solutions and comparison to predictions of the DLVO theory. J. Colloid Interface Sci. 2010, 342, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Varoqui, R. Effect of polymer adsorption on the electrophoretic mobility of colloids. New J. Chem. 1998, 6, 187–189. [Google Scholar]
- Salemis, P. Caractérisation des Amidons. Application à L'étude du Mécanisme de Flottation des Minerais. Ph.D. Thesis, Joseph Fourier University, Grenoble, France, November 1984. [Google Scholar]
- Hoeve, C.A.J.; DiMarzio, E.A.; Peyser, P. Adsorption of polymer molecules at low surface coverage. J. Chem. Phys. 1965, 42, 2558–2563. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Bhardwaj, N.K.; Choushary, V. Synthesis and characterization of methylcellulose/PVA based porous composite. Carbohydr. Polym. 2012, 88, 1364–1372. [Google Scholar] [CrossRef]
- Culminal. Methylcellulose, Methylhydroxyethylcellulose, Methylhydroxypropylcellulose. Physical and Chemical Properties. Available online: http://www.brenntagspecialties.com/en/downloads/Products/Multi_Market_Principals/Aqualon/Culminal_MC_Booklet.pdf (accessed on 20 April 2015).
- Methyl Cellulose. Available online: http://en.wikipedia.org/wiki/Methyl_cellulose (accessed on 20 April 2015).
- Noronha, C.M.; Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcelluloses film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Podczeck, F.; Jones, B.E. Gelatin alternatives and additives. In Pharmaceutical Capsules, 2nd ed.; Pharmaceutical Press: London, UK, 2004; Volume 3, pp. 61–63. [Google Scholar]
- Sandford-Smith, J. Eye Diseases in Hot Climates, 2nd ed.; Butterworth-Heunemann Ltd.: Guilford, UK, 1990. [Google Scholar]
- Stewart, G.J.; Wang, Y.; Niewiarowski, S. Methylcellulose protects the ability of anchorage-dependent cells to adhere following isolation and holding in suspension. Biotechniques 1995, 19, 598–604. [Google Scholar] [PubMed]
- Reamer, R.; Dey, B.P.; Thaker, N. Cryopreservation of bacterial vegetative cells used in antibiotic assay. J. AOAC Int. 1994, 78, 997–1001. [Google Scholar]
- Freedman, V.H.; Shin, S.I. Cellular tumorigenicity in nude mice: Correlation with cell growth in semi-solid medium. Cell 1974, 3, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Risser, R.; Pollack, R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology 1974, 59, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Müller-Sieburg, C.E.; Townsend, K.; Weissman, I.L.; Rennick, D. Proliferation and differentiation of highly enriched mouse hematopoietic stem cells and progenitor cells in response to defined growth factors. J. Exp. Med. 1988, 167, 1825–1840. [Google Scholar] [CrossRef] [PubMed]
- Rennick, D.; Yang, G.; Muller-Sieburg, C.; Smith, C.; Arai, N.; Takabe, Y.; Gemmell, L. Interleukin 4 (B-cell stimulatory factor 1) can enhance or antagonize the factor-dependent growth of hemopoietic progenitor cells. Proc. Natl. Acad. Sci. USA 1987, 84, 6889–6893. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishikawa, Y.; Okano, S. Cloning of human neuroblastoma cells in methylcellulose culture. Cancer Res. 1987, 47, 4146–4149. [Google Scholar] [PubMed]
- Nathan, M.; Homm, R.E. Methyl Cellulose Sponge and Method of Making. US Patent No. 3,005,457, 24 October 1961. [Google Scholar]
- Vieira, J.G.; Filho, G.R.; Meireles, C.S.; Faria, F.A.C.; Gomide, D.D.; Pasquini, D.; Cruz, S.F.; Assunção, R.M.N.; Motta, L.A.C. Synthesis and characterization of methylcellulose from cellulose extracted from mango seeds for use as a mortar additive. Polímeros 2012, 22, 80–87. [Google Scholar] [CrossRef]
- Walocel Methylcellulose for Cement Render: Robust Performance in a Range of Cement Qualities. Available online: http://www.dowconstructionchemicals.com/eu/en/pdf/840-02201.pdf (accessed on 20 April 2015).
- Dow Industrial Specialities: Applications. Available online: http://www.dow.com/dowwolff/en/industrial_solutions/application/pvc.htm (accessed on 20 April 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015, 7, 777-803. https://doi.org/10.3390/polym7050777
Nasatto PL, Pignon F, Silveira JLM, Duarte MER, Noseda MD, Rinaudo M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers. 2015; 7(5):777-803. https://doi.org/10.3390/polym7050777
Chicago/Turabian StyleNasatto, Pauline L., Frédéric Pignon, Joana L. M. Silveira, Maria Eugênia R. Duarte, Miguel D. Noseda, and Marguerite Rinaudo. 2015. "Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications" Polymers 7, no. 5: 777-803. https://doi.org/10.3390/polym7050777
APA StyleNasatto, P. L., Pignon, F., Silveira, J. L. M., Duarte, M. E. R., Noseda, M. D., & Rinaudo, M. (2015). Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers, 7(5), 777-803. https://doi.org/10.3390/polym7050777