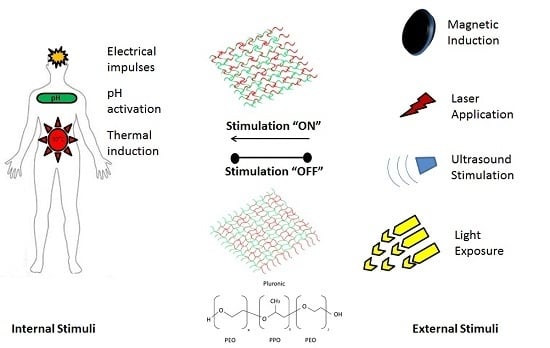
A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents
Abstract
:1. Introduction
2. Advantages and Mechanisms of Cancer Targeting via Thermoresponsive Systems
3. Advantages and Stimulation Mechanism of Ultrasound-Responsive Polymers in Cancer Therapy
4. Overview of the Properties and Functionality of Diverse Thermo- and Ultrasound-Responsive Systems in Cancer Therapy
4.1. Properties of Thermoresponsive Polymer Systems: Temperature Ranges at Phase Transitions
4.2. Modification of Thermoresponsive System Properties for Enhanced Cancer Therapy
4.3. Advancing Functional Trends in Thermoresponsive Polymers
4.4. Modification of Ultrasound-Responsive System Properties for Enhanced Cancer Therapy
5. Pseudo-Ultrasound-Responsive Systems in Cancer Chemotherapeutics: Advancing Trends in Ultrasound-Responsive Systems
6. An Overview of Various Chemotherapeutic Delivery Systems Combining Thermal- and Ultrasound-Responsive Polymers
7. Conclusions and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Abbreviation | Compound Name |
| AA | Acrylic Acid |
| AAm | Acrylamide |
| PBMA | Poly(Butyl Methacrylate) |
| BSA | Bovine Serum Albumin |
| CHC | Carboxymethyl-Hexanoyl Chitosan |
| CHOL | Cholesterol |
| DMDEA | 2-(5,5-Dimethyl-1,3-Dioxan-2 yloxy) Ethyl Acrylate |
| DPPC | 1,2-Dipalmitoyl-sn-glycero-3-Phosphatidylcholine |
| DPPE | Dipalmitoyl-sn-glycero-3-Phosphatidylethanolamine |
| DPPG | Dipalmitoyl Phosphatidylglycerol |
| DPSC | 1,2-Distearoyl-sn-glycero-Phosphcholine |
| DPSE | 1,2 Distearoyl-sn-glycero-3-Phosphoethanolamine |
| DSPC | 1,2-Distearoyl-sn-glycero-3-Phosphotidylcholine |
| DSPE | 1,2-Distearoyl-sn-glycero-3-Phosphoethanolamine |
| DSPG | 1,2-Dioctadecanoyl-sn-glycero-3-Phospho-(1′-rac-glycerol) |
| HAP-1 | Human Atherosclerotic Peptide-1 |
| HMM | 2-Hydroxymethyl Methacrylate |
| MOEGA | Monomethyl Oligo (Ethylene Glycol) Acrylate |
| MSPC | 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine |
| N-DMAM | N-Dimethylacrylamide |
| N-(HM)A | N-Hydroxymethyl Acrylamide |
| N′N DMAM | N,N-Dimethylacrylamide |
| P(βA) | Poly(β Benzyl-l-Aspartate) |
| P(THFMA) | Poly(2-Tetrahydrofuranoloxy) Ethylmethacrylate |
| P(THPMA) | Poly(2-Tetrahydropyranyl) Methacrylate |
| PCL | Poly-ε-Caprolactone |
| PEG | Polyethylene Glycol |
| PEG'd | Polyethylene Glycolated |
| PEI | Polyethyleneamine |
| PEO | Polyethylene Oxide |
| PIBMA | Poly[(Isobutoxy) Ethyl Methacrylate] |
| PFC | Perfluorocarbon |
| PFH | Perfluorohexane |
| PFP | Perfluoropentane |
| PLA | Poly(Lactide)/Poly(Lactic Acid) |
| PLGA | Poly(d,l-Lactide-co-Glycolide) |
| PLLA | Pol-l-Lysine |
| PMA | Poly(Methacrylate) |
| PMMA | Poly(Methyl Methacrylate) |
| P(NIPAM) | Poly(N-Isopropyl Acrylamide) |
| SPIO | Super Paramagnetic Iron Oxide |
References
- Deckers, R.; Moonen, C.T.W. Ultrasound triggered, image guided, local drug delivery. J. Control. Release 2010, 148, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yudina, A.; Lepetit-Coiffé, M.; De Smet, M.; Langereis, S.; Grüll, H.; Moonen, C. In vivo temperature controlled ultrasound-mediated intracellular delivery of cell-impermeable compounds. J. Control. Release 2012, 161, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, F.; Cheng, R.; Denga, C.; Feijen, J.; Zhong, Z. Advanced drug and gene delivery systems based on functional biodegradable polycarbonates and copolymers. J. Control. Release 2014, 190, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Qui, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide) coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lim, L.H.; Wang, Q.; Jiang, S.P. Thermoreversible micellization and gelation of a blend of pluronic polymers. Polymer 2008, 49, 1952–1960. [Google Scholar] [CrossRef]
- Yannic, B.; Schuetz, Y.B.; Gurny, R.; Jordan, O. A novel thermoresponsive hydrogel based on chitosan. Eur. J. Pharm. Biopharm. 2008, 68, 19–25. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Banerjee, S.; Chaurasia, G.; Ghosh, A. Smart polymers: Around the cosmos. Asian J. Pharm. Clin. Res. 2010, 3, 135–141. [Google Scholar]
- Nazar, H. Using “smart” biomaterials and systems for targeted drug delivery. Pharm. J. 2013, 290, 646. [Google Scholar]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.A.; Palalon, F.; Sanders, K.E.; Koshkina, N.V. The effects of non-invasive radiofrequency treatment and hyperthermia on malignant and nonmalignant cells. Int. J. Environ. Res. Public Health 2014, 11, 9142–9153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-L.; Chen, Z.-Y.; Wang, Y.-X.; Yang, F.; Lin, Y.; Liao, Y.-Y. Ultrasound-mediated local drug and gene delivery using nanocarriers. BioMed Res. Int. 2014, 2014, 963891. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- You, J.-O.; Almeda, D.; Ye, G.J.C.; Auguste, D.T. Bioresponsive matrices in drug delivery. J. Biol. Eng. 2010, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Saphwan Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering-From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 119–150. [Google Scholar]
- Iyer, A.K.; Singh, A.; Ganta, S.; Amiji, M.M. Role of integrated cancer nanomedicine in overcoming drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1784–1802. [Google Scholar] [CrossRef] [PubMed]
- Kiminta, D.M.O.; Braithwaite, G.; Luckham, P.F. Colloid Dispersions, nanogels. In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 259–261. [Google Scholar]
- Chen, Y.-Y.; Wu, H.-C.; Sun, J.-S.; Dong, G.-C.; Wang, T.-W. Injectable and Thermoresponsive Self-assembled nanocomposite hydrogel for long-term anticancer drug delivery. Langmuir 2013, 29, 3721–3729. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Kröger, M. Collapse of thermoresponsive brushes and the tuning of protein adsorption. Macromolecules 2011, 44, 6986–7005. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M. Theoretical considerations on mechanisms of harvesting cells cultured on thermoresponsive polymer brushes. Biomaterials 2012, 33, 4975–4987. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hu, H.; Chen, L.; Bao, X.; Li, Y.; Chen, L.; Xu, G.; Yeb, X.; Ding, J. Comparative studies of thermogels in preventing post-operative adhesions and corresponding mechanisms. Biomater. Sci. 2014, 2, 1100–1109. [Google Scholar] [CrossRef]
- Galaev, I.; Mattiasson, B. Smart Polymers: Applications in Biotechnology and Biomedicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 144–146. [Google Scholar]
- Ci, T.; Chen, L.; Yu, L.; Ding, J. Tumor regression achieved by encapsulating a moderately soluble drug into a polymeric thermogel. Sci. Rep. 2014, 4, 5473. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.M.; De Oliveira, P.C.; Dos Santos, A.M.; Ambrosio Zanin, M.H.; Inês Ré, M. Synthesis and characterization of thermo-responsive particles of poly(hydroxybutirate-co-hydroxyvalerate)-b-poly(N-isopropylacrylamide). Braz. J. Phys. 2009, 39, 217–222. [Google Scholar] [CrossRef]
- Couture, O.; Foley, J.; Kassell, N.F.; Larrat, B.; Aubry, J.-F. Review of ultrasound mediated drug delivery for cancer treatment: Updates from pre-clinical studies. Transl. Cancer Res. 2014, 3, 494–511. [Google Scholar]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [PubMed]
- Phenix, C.P.; Togtema, M.; Pichardo, S.; Zehbe, I.; Curiel, L. High Intensity Focused Ultrasound technology, its scope and applications in therapy and drug delivery. J. Pharm. Sci. 2014, 17, 136–153. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Fite, B.Z.; Ferrara, K.W. Ultrasonic enhancement of drug penetration in solid tumours. Front. Oncol. 2013, 3, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Legay, M.; Gondrexon, N.; Person, S.L.; Boldo, P.; Bontemps, A. Enhancement of heat transfer by ultrasound: Review and recent advances. Int. J. Chem. Eng. 2011, 2011, 670108. [Google Scholar] [CrossRef]
- Peteu, S.F.; Oancea, F.; Sicuia, O.A.; Constantinescu, F.; Dinu, S. Responsive polymers for crop protection. Polymers 2010, 2, 229–251. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Du, L.-N.; Lu, C.-T.; Jin, Y.-G.; Ge, S.P. Potential and problems in ultrasound-responsive drug delivery systems. Int. J. Nanomed. 2013, 8, 1621–1633. [Google Scholar]
- Mao, Z.; Maa, L.; Yana, J.; Yan, M.; Gao, C.; Shen, J. The gene transfection efficiency of thermoresponsive N,N,N-trimethyl chitosan chloride-g-poly(N-isopropylacrylamide) copolymer. Biomaterials 2007, 28, 4488–4500. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-S. Design and development of degradable polyethylenimines for delivery of DNA and small interfering RNA: An updated review. Mater. Sci. 2012, 2012, 798247. [Google Scholar] [CrossRef]
- Lai, B.F.L.; Zou, Y.; Yang, X.; Yu, X.; Kizhakkedathu, J.N. Abnormal blood clot formation induced by temperature responsive polymers by altered fibrin polymerization and platelet binding. Biomaterials 2014, 35, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, R.; Zou, C.; Yang, D.; Chen, Z.-S. Fragmented polymer nanotubes from sonication induced scission with a thermo-responsive gating system for anti-cancer drug delivery. J. Mater. Chem. B 2014, 2, 1327–1334. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Nabid, M.R.; Niknejad, H.; Entezami, A.A. Multifunctional and thermoresponsive unimolecular micelles for tumor-targeted delivery and site-specifically release of anticancer drugs. Polymer 2012, 53, 3485–3497. [Google Scholar] [CrossRef]
- James, H.P.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Abulateefeh, S.R.; Spain, S.G.; Aylott, J.W.; Chan, W.C.; Garnett, M.C.; Alexander, C. Thermoresponsive polymer colloids for drug delivery and cancer therapy. Macromol. Biosci. 2011, 11, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Chen, Y.; Fuchise, K.; Takada, K.; Kitakado, J.; Sato, S.-I.; Satoh, T.; Kakuchi, T. Thermoresponsive properties of 3-, 4-, 6-, and 12-armed star-shaped poly[2-(dimethylamino)ethyl methacrylate]s prepared by core-first group transfer polymerization. Polym. Chem. 2014, 5, 4701–4709. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent Advances in Shape Memory Soft Materials for Biomedical Applications. Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Jung, S.-H.; Jeonga, H.M.; Lee, H.-I. Thermoresponsive ureido-derivatized polymers: The effect of quaternization on UCST properties. Polym. Chem. 2014, 5, 2411–2416. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Li, Z.; Gao, G.; Liu, F. Temperature-responsive properties of poly(acrylic acid-co-acrylamide) hydrophobic association hydrogels with high mechanical strength. Macromolecules 2010, 43, 10645–10651. [Google Scholar] [CrossRef]
- Ray, D.; Mohapatra, D.K.; Maohapatra, R.K.; Mohanta, G.P.; Sahoo, P.K. Synthesis and colon-specific drug delivery of a poly(acrylic acid-co-acrylamide)/MBA nanosized hydrogel. J. Biomater. Sci. Polym. Ed. 2008, 19, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mijalis, A.J.; Fu, H.; Agosto, C.; Tan, K.J.; Batteas, J.D.; Bergbreiter, D.E. Reversible changes in solution pH resulting from changes in thermoresponsive polymer solubility. J. Am. Chem. Soc. 2012, 134, 7378–7383. [Google Scholar] [CrossRef] [PubMed]
- Fuchise, K. Design and Precise Synthesis of Thermoresponsive Polyacrylamides. Ph.D. Thesis, Hokkaido University, Sapporo, Japan, 2014. [Google Scholar]
- Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, S.; Chang, P.E.J.; Rumpel, H.; Kee, I.H.C.; Ng, R.T.H.; Chow, P.H.K.; Tan, C.K.; Ramanujan, R.V. Thermoresponsive core–shell magnetic nanoparticles for combined modalities of cancer therapy. Nanotechnology 2015, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Liu, Y.; Zhao, J.; Zhou, M.; Bouchard, R.R.; Mitchama, T.; Wallace, M.; Stafford, R.J.; Li, C.; Gupta, S.; et al. In vitro and vivo of mapping of drug release after laser ablation thermal therapy with doxorubicin-loaded hollow gold nanoshells using fluorescence and photoacoustic imaging. J. Control. Release 2013, 172, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Menon, J.U.; Tsai, Y.S.; Gore, C.; Dobin, T.; Gandee, L.; Kangasniemi, K.; Takahashi, M.; Manandhar, B.; Ahn, J.M.; et al. Prostate cancer-specific thermo-responsive polymer-coated iron oxide nanoparticles. Biomaterials 2013, 34, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xiang, X.; Heiden, P.A. Tuning thermoresponsive behavior of diblock copolymers and their gold core hybrids. Part 2. How properties change depending on block attachment to gold nanoparticles. J. Colloid Interface Sci. 2013, 396, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Okano, T. Polymer terminal group effects on properties of thermoresponsive polymeric micelles with controlled outer-shell chain lengths. Biomacromolecules 2005, 6, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Rezaeia, S.J.T.; Nabida, M.R.; Niknejadb, H.; Entezami, A.A. Folate-decorated thermoresponsive micelles based on star-shaped amphiphilic block copolymers for efficient intracellular release of anticancer drugs. Int. J. Pharm. 2012, 437, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Webster, D.C.; Singh, J. Thermosensitive polymers: Synthesis, characterization, and delivery of proteins. Int. J. Pharm. 2007, 341, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Ghosh, R. Thermo-responsive hydrogels for stimuli-responsive membranes. Processes 2013, 1, 238–262. [Google Scholar] [CrossRef]
- Shao, P.; Wang, B.; Wang, Y.; Li, J.; Zhang, Y. The application of thermosensitive nanocarriers in controlled drug delivery. J. Nanomater. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Haidar, Z.S. Bio-inspired/-functional colloidal core–shell polymeric-based nanosystems: Technology promise in tissue engineering, bioimaging and nanomedicine. Polymers 2010, 2, 323–352. [Google Scholar] [CrossRef]
- Kokardekar, R.R.; Sha, V.K.; Mody, H.R.; Hiranandani, L.H. PNIPAM Poly (N-isopropylacrylamide): A thermoresponsive “smart”polymer in novel drug delivery systems. Int. J. Med. Update 2012, 7, 60–63. [Google Scholar]
- Chang, B.; Sha, X.; Guo, J.; Jiao, Y.; Wang, C.; Yang, W. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J. Mater. Chem. 2011, 21, 9239–9247. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M. Thermoresponsive cell culture substrates based on PNIPAM brushes functionalized with adhesion peptides: Theoretical considerations of mechanism and design. Langmuir 2012, 28, 16623–16637. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wang, W.; Wang, C.; Wang, Y.; Zhou, J.; Din, Y.; Wang, X.; Jin, Y. A chitosan-graft-PEI-candersartan conjugate for targeted co-delivery of drug and gene in anti-angiognesis cancer therapy. Biomaterials 2014, 35, 8450–8466. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mackay, J.A.; Dreher, M.R.; Chen, M.; McDaniel, J.R.; Simnick, A.J.; Callahan, D.J.; Zalutsky, M.R.; Chilkoti, A. Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model. J. Control. Release 2010, 144, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Chaw, C.S.; Chooi, K.W.; Liu, X.M.; Tan, C.W.; Wang, L.; Yang, Y.Y. Thermally responsive core–shell nanoparticles self-assembled from cholesteryl end-capped and grafted polyacrylamides: Drug incorporation and in vitro release. Biomaterials 2004, 25, 4297–4308. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tong, Y.W.; Yang, Y.Y. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(d,l-lactide-co-glycolide) with varying compositions. Biomaterials 2005, 26, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Kohori, F.; Sakai, K.; Aoyagi, T.; Yokoyama, M.; Yamato, M.; Sakurai, Y.; Pkano, T. Control of Adriamycin cytotoxic activity using thermally responsive polymeric micelles composed of poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)b-poly(d,l-lactide). Colloids Surf. B 1999, 16, 195–205. [Google Scholar] [CrossRef]
- Meyer, D.E.; Shin, B.C.; Kong, G.A.; Dewhirst, M.W.; Chikoti, A. Drug targeting using thermally responsive polymers and local hyperthermia. J. Control. Release 2001, 74, 213–224. [Google Scholar] [CrossRef]
- Strong, L.E.; Dahorte, S.N.; West, J.L. Hydrogel-nanoparticle composites for optically modulated cancer therapeutic delivery. J. Control. Release 2014, 178, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.Y.; Zhang, R.; Du, F.S.; Liang, D.H.; Li, Z.C. Multi-responsive nanogels containing motifs of ortho ester, oligo (ethylene glycol) and disulphide linkage as carriers of hydrophobic anti-cancer drugs. J. Control. Release 2011, 152, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Okano, T.; Miyakazi, T.; Kohori, F.; Sakai, K.; Yokoyama, M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J. Control. Release 2006, 115, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Zhang, W.; Poventud-Fuentes, I.; Wang, Y.; Lei, Y.; Agarwal, P.; Weekes, B.; Li, C.; Lu, X.; Yu, J.; et al. Thermally responsive nanoparticle-encapsulated curcumin and its combination with mild hyperthermia for enhanced cancer cell destruction. Acta Biomater. 2004, 10, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, S.; Ramanujan, R.V. Thermoresponsive magnetic composite nanomaterials for multimodal cancer therapy. Acta Biomater. 2010, 6, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Yahuafai, J.; Asaia, T.; Nakamura, G.; Fukuta, T.; Siripong, P.; Hyodo, K.; Ishihara, H.; Kikuchi, H.; Oku, N. Suppression of mice of immunosurveillance against PEGylated liposomes by encapsulated doxorubicin. J. Control. Release 2014, 192, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.N.; Zheng, J.; Foltz, W.D.; Weersink, R.; Chaudary, N.; Jaffay, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; May, J.P.; Ernsting, M.J.; Li, S.D. A thermosensitive liposome prepared with a Cu2+ gradient demonstrates improved pharmacokinetics, drug delivery and antitumor efficacy. J. Control. Release 2012, 161, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-responsive polymer nanoparticles with a core–shell micelle structure as site-specific drug carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, W.; Hu, H.; Mac, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control. Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.C.; Zhang, X.; Lu, W.L.; Zhang, Q. A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J. Control. Release 2009, 135, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Dicheva, B.M.; Ten Hagen, T.L.M.; Schipper, D.; Seynhaeve, A.L.B.; Von Rhoon, G.C.; Eggermont, A.M.M.; Koning, G.A. Targeted heat-triggered doxorubicin delivery to tumors by dual targeted cationic thermosensitive liposomes. J. Control. Release 2014, 195, 37–48. [Google Scholar] [CrossRef] [PubMed]
- De Smet, M.; Langereis, S.; Van den Bosck, S.; Grüll, H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. J. Control. Release 2010, 143, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plack, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Y.; Wang, Z.; Huang, S.; Chen, Y.; Jiang, W.; Zhang, H.; Ding, M.; Li, Q.; Xiao, X.; et al. Methotrexate-loaded PLGA nanobubbles for ultrasound imaging and Synergistic Targeted therapy of residual tumor during HIFU ablation. Biomaterials 2014, 35, 5148–5161. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Okada, K.; Yamamoto, K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys. J. 2009, 96, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.J.; Wan, J.J.; Qiao, Y.-Z.; Li, W.S.; Zou, X.R.; Wan, M.X. Cavitation endothelium damage of large artey vessel: The application to animal model of atheresclerosis. In The International Conference on Health Informatics; Zhang, Y.-T., Ed.; Springer: Cham, Switzerland, 2014; Volume 42, pp. 63–66. [Google Scholar]
- Fabiilli, M.L.; Haworth, K.J.; Sebastian, I.E.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. Delivery of chlorambucil using an acoustically-triggered, perfluoropentane emulsion. Ultrasound Med. Biol. 2010, 36, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, D.; Jin, S.; Ding, J.; Guo, J.; Shi, W.; Wang, C. Stimuli-responsive biodegradable poly(methacrylic acid) based nanocapsules for ultasound traced and triggered drug delivery system. Biomaterials 2014, 35, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Gourevich, D.; Dogadkin, O.; Volovick, A.; Wang, L.; Gnaim, J.; Cochran, S.; Melzer, A. Ultrasound-mediated targeted drug delivery with a novel cyclodextrin-based drug carrier by mechanical and thermal mechanisms. J. Control. Release 2013, 170, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Yamashita, T.; Kawabata, S.; Shimizu, N. Targeted and ultrasound-triggered drug delivery using liposomes co-modified with cancer cell-targeting aptamers and a thermosensitive polymer. Ultrason. Sonochem. 2014, 21, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Wong, T.T.; Teng, M.C.; Liu, R.S.; Lu, M.; Liang, H.F.; Wei, M.C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Husseini, G.A.; Pitt, W.G. The use of ultrasound and micelles in cancer treatment. J. Nanosci. Nanotechnol. 2008, 8, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Payne, A.; Dillon, C.; Shea, J.; Scaife, C.; Gupta, R. Focused ultrasound-mediated drug delivery to pancreatic cancer in a mouse model. J. Ther. Ultrasound 2013, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, L.; Deng, Z.; Jin, Q.; Chen, J.; Yang, W.; Yeh, C.; Wu, J.; Shandas, R.; Liu, X.; et al. Paclitaxel-liposome–microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J. Control. Release 2013, 166, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Ting, C.Y.; Lin, H.J.; Wang, C.H.; Liu, H.L.; Yen, T.C.; Yeh, C.K. SPIO-conjugated, doxorubicin loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials 2013, 34, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Ting, C.Y.; Lin, H.J.; Wang, C.H.; Liu, H.L.; Yen, T.C.; Yeh, C.K. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials 2013, 34, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Lui, T.Y.; Wu, M.Y.; Lin, M.H.; Yang, F.Y. A novel ultrasound-triggered drug vehicle with multimodal imaging functionality. Acta Biomater. 2013, 9, 5453–5463. [Google Scholar]
- Geers, B.; Lentacker, I.; Sanders, N.N.; Demeester, J.; Meairs, S.; De Smedt, S.C. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. J. Control. Release 2011, 152, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochran, M.C.; Einsbery, J.; Ouma, R.O.; Soulen, M.; Wheatley, M.A. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm. 2011, 414, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Einsbery, J.R.; Burnstein, O.M.; Kambhampati, R.; Forsberg, F.; Lui, J.B. Development and optimization of a doxorubicin loaded poly(lactic acid) contrast agent for ultrasound directed drug delivery. J. Control. Release 2010, 143, 38–44. [Google Scholar]
- Tinkov, S.; Winter, G.; Coester, C.; Bekeredjian, R. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: Part I—Formulation development and in vitro characterization. J. Control. Release 2010, 143, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, Y.; Ran, H.; Tan, J.; Lin, Y.; Zhang, Q.; Ren, J.; Wang, Z. Ultrasound triggered drug release from 10-hydroxycamptothecin-loaded phospholipid microbubbles for targeted tumor therapy in mice. J. Control. Release 2012, 162, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tong, S.; Dewhirst, M.W.; Yuan, F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol. Cancer Ther. 2004, 3, 1311–1317. [Google Scholar] [PubMed]
- Yudina, A.; De Smet, M.; Lepetit-Coiffé, M.; Langereis, S.; Van Ruijssevelt, L.; Smirnov, P.; Bouchaud, V.; Voisin, P.; Grüll, H.; Moonen, C.T.W. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J. Control. Release 2010, 155, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Oda, Y.; Utoguchi, N.; Namai, E.; Taira, Y.; Okada, N.; Kadowaki, N.; Kodama, T.; Tachibana, K.; Maruyama, K. A novel strategy utilizing ultrasound for antigen delivery in dendritic cell-based cancer immunotherapy. J. Control. Release 2009, 133, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Namai, E.; Oda, Y.; Nishiie, N.; Otake, S.; Koshima, R.; Hirata, K.; Taira, Y.; Utoguchi, N.; Negishi, Y.; et al. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J. Control. Release 2010, 142, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, J.D.; Pitt, W.G. Sequestration and ultrasound-induced release of doxorubicin from stabilized Pluronic P105 micelles. Drug Deliv. 2002, 9, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Oh, S.H.; Seo, T.B.; Namgung, U.; Kim, J.M.; Lee, J.H. Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. J. Biomed. Mater. Res. B 2010, 94, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pelletier, M.; Zhang, H.; Xia, H.; Zhao, Y. High-frequency ultrasound-responsive block copolymer micelle. Langmuir 2009, 25, 13201–13205. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, L.Y. Sun, nanotechnology applied to overcome tumor drug resistance. J. Control. Release 2012, 162, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Stover, T.C.; Kim, Y.S.; Lowe, T.L.; Kester, M. Thermoresponsive and biodegradable linear-dendritic nanoparticles for targeted and sustained release of a pro-apoptotic drug. Biomaterials 2008, 29, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.Y.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 2009, 138, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.G.; Fain, H.D.; Rapoport, N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J. Control. Release 2005, 102, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Honen, R.; Turjeman, K.; Gabizon, A.; Kost, J.; Barenholz, Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J. Control. Release 2009, 137, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- Lin, H.Y.; Thomas, J.L. Factors affecting responsivity of unilamellar liposomes to 20 kHz ultrasound. Langmuir 2004, 20, 6100–6106. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Du, J. Ultrasound and pH dually responsive polymer vesicles for anticancer drug delivery. Sci. Rep. 2013, 3, 2162. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Gao, Z.; Lee, S.W.; Seo, M.H.; Rapoport, N. Ultrasound-enhanced chemotherapy of drug-resistant breast cancer tumors by micellar-encapsulated Paclitaxel. Am. J. Drug Deliv. 2006, 4, 97–104. [Google Scholar] [CrossRef]
- Ayano, E.; Karki, T.; Kanawaza, H.; Okano, T. Poly(N-isopropylacrylamide)-PLA and PLA blend nanoparticles for temperature-controllable drug release and intracellular uptake. Colloids Surf. B 2012, 99, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pitt, W.G. A polymeric micelle system with a hydrolysable segment for drug delivery. J. Biomater. Sci. Polym. Ed. 2006, 17, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Pelletier, M.; Xia, H.; Zhao, Y. Ultrasound-induced disruption of amphiphilic block copolymer micelles. Macromol. Chem. Phys. 2011, 212, 498–506. [Google Scholar] [CrossRef]
- Gao, Z.; Kennedy, A.M.; Christensen, D.A.; Rapoport, N.Y. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 2008, 48, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Bartolak-Suki, E.; Park, E.J.; Karrobi, K.M.; McDannold, N.J.; Porter, T.M. Localized delivery of doxorubicin in vivo from polymer-modified thermosensitive liposomes with MR-guided focused ultrasound-mediated heating. J. Control. Release 2014, 194, 71–81. [Google Scholar] [CrossRef] [PubMed]







| Thermoresponsive system | Bioactive | Cancer type | Switching temperature | Reference |
|---|---|---|---|---|
| P(NIPAM-co-AA) | Doxorubicin | Breast Cancer | 5–50 °C | [48] |
| P(NIPAM) | Doxorubicin | Multimodal Cancer | 33.4 °C, 38.3 °C | [53] |
| PEG-HAuNS | Doxorubicin | Breast Cancer | [54] | |
| P(NIPAM-acrylamide-allylamine) | - | Prostate Cancer | 40 °C | [55] |
| Chitosan-g-PEI | Bafilomycin Candesartan | Pancreatic Cancer | - | [66] |
| Elastin-Like peptide | Radionuclide | Advanced Stage Tumoral Cancers | 27 °C, 62.5 °C | [67] |
| P(NIPAM-co-N,NDMAM), CHOL-g-P[NIPAM-co-N-(hydroxymethyl) acrylamide] | Indomethacin | - | - | [68] |
| P(NIPAM-co-N,NDMAM)-b-PLGA | Doxorubicin | Cancer | 39.5 °C | [69] |
| P(NIMAM-co-N,NDMAM)-b-PLa | Adriamycin | Bovine Aorta Endothelial | 40 °C | [70] |
| P(NIPAM-co-AAm) | No drug (rhodamine); Doxorubicin | Ovarian Cancer; Colon Cancer | 40 °C, >0 °C | [71,72] |
| P(MOEGA–DMDEA) | Paclitaxel, Doxorubicin | - | 23.8–35.1 °C | [73] |
| P(NIPAM-co-N,NDMAM)-b-P(LA-co-CL) | Doxorubicin | - | ~40 °C | [74] |
| P(NIPAM-b-PBMA) | Adriamycin | - | 32.5 °C | [75] |
| Pluronic F127–chitosan | Curcumin | Prostate Cancer | 37 °C | [76] |
| (Fe3O4)-P(NIPAM) | Doxorubicin | Cancer | 35 °C | [77] |
| PEG′d Liposomes | Doxorubicin | Liver Cancer | - | [78] |
| DPPC:DPPG:MSPC:mPEG2000-DSPE (HTLC) | Cisplatin | Cervical Cancer | 40–43 °C | [79] |
| DPPC/MSPC/DSPE-PEG2000 | Breast Cancer | 37–42 °C | [80] | |
| P(NIPAM)-NH2(Amino-terminated) | Adriamycin | - | 32 °C | [81] |
| Pluronic 127 | Paclitaxel, Docetaxel | Breast Cancer, Ovarian Cancer | >20 °C, 21 °C | [82,83] |
| DPPC:DSPC:DPTAP:DSPE:PEG2000 | Doxorubicin | Lung Carcinoma | ~39 °C | [84] |
| DPPC:MPPC:DPPE-PEG2000, DPPC:HSPC:CHOL:DPPE-PEG2000 | [Gd(HPDO3A)(H2O)] + Doxorubicin | Squamous Cell Carcinoma | - | [85] |
| DPPC:CHOL:DSPE-PEG2000:DSPE-PEG2000-Folate | Doxorubicin | Epidermoid Cancer, Cervical Cancer | 41.6 °C | [86] |
| Ultrasound-Responsive system | Bioactive | Cancer type | Reference |
|---|---|---|---|
| PLGA | Methotrexate | Placental Chariocarcinoma | [87] |
| PMMA | Doxorubicin | Liver Cancer | [92] |
| γCyclodextran-N-Boc-3-(2-naphthyl)-alanine | Doxorubicin | Breast Cancer | [93] |
| APT:P(NIPAM-co-NIPAM) | Doxorubicin | Breast Cancer | [94] |
| l-α-phosphatidylcholine:CHOLl:DSPE-PEG2000 | Doxorubicin | Glioblastoma | [95] |
| PDA:PDLA-PFC | Paclitaxel | Pancreatic Cancer | [97] |
| Pluronic | Doxorubicin | - | [96] |
| (DSPE-PEG2000-Biotin) | Paclitaxel | Breast Cancer | [98] |
| DSPC:DSPE-PEG2000:DSPG | Doxorubicin | Brain Cancer | [99] |
| DPPC:DPSE:PEG2000:Biotin | 1,3-bis(2-chloroethyl)-1-nitrosourea | Glioma (Brain/Spinal Tumor) | [100] |
| CHC:SPIO:BSA (MB) | Camptothecan | Breast Tumour Cells | [101] |
| DPPC-DSPE-PEG-maleimide:CHOL | Doxorubicin | Lung Cancer | [102] |
| PLA | Paclitaxel, Doxorubicin | Breast Cancer, Liver Cancer | [103,104] |
| DPPC:DPPG:DPPE-PEG2000 | Doxorubicin | Cancer | [105] |
| DPPC:DPPA:DPPE-PEG2000 | 10-Hydroxycamptothecin | Liver Cancer | [106] |
| Lysolecithin-containing DPPC:MSPC:DSPE-PEG2000 | Doxorubicin | Squamous Cell Carcinoma | [107] |
| DPPC:MSPC:DSPE-PEG2000 | TO-PRO-3 (DNA-intercalating agent) | Glioma | [108] |
| DSPC: DSPE-PEG(2k)-OMe | HLA (Antigen) | - | [109] |
| DSPC: DSPE-PEG(2k)-OMe | IL-12 | Ovarian Cancer | [110] |
| Thermoresponsive component | Ultrasound-Responsive component | Bioactive | Cancer type | References |
|---|---|---|---|---|
| N,N-DEAM | Pluronic | Doxorubicin | Leukaemia (HL-60) | [111] |
| P(NIPAM)-co-N,NDMAM | PLGA | Doxorubicin | Breast Cancer | [112] |
| PEO | Poly(THPMA) | - | - | [113] |
| P(NIPAM)-PLA | PLLA-PLA | Sphingolipid ceramide | Cancer | [115] |
| PEO | PLA | Paclitaxel | Pancreatic, Ovarian, Breast Cancer Tumours | [116] |
| PEG2000-diacylphospholipid | Pluronic P-105 | Doxorubicin | Ovarian Cancer | [117] |
| PEG | P(βA) | Doxorubicin | Ovarian Cancer | [117] |
| PE: DSPE | CHOL | Cisplatin | Colon Cancer | [119] |
| DPPC | (DPPE-PEG2000) | Calcein | - | [120] |
| PEO | P(2-THFMA) | Doxorubicin | Liver Cancer | [121] |
| PEO | PLA-tocopherol | Paclitaxel | Breast Cancer | [122] |
| P(NIPAM) | PLA | - | - | [123] |
| PEO, P(NIPAM) | 2-hydroxymethyl methacrylate | Doxorubicin | - | [124] |
| PEO | PIBMA, P(2-THMA) | - | - | [125] |
| PEO | PLA, PCL | Doxorubicin | Breast Cancer | [126] |
| P(NIPAM-co-PAA) | Propylacrylic acid | Doxorubicin | Breast Cancer | [127] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zardad, A.-Z.; Choonara, Y.E.; Du Toit, L.C.; Kumar, P.; Mabrouk, M.; Kondiah, P.P.D.; Pillay, V. A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents. Polymers 2016, 8, 359. https://doi.org/10.3390/polym8100359
Zardad A-Z, Choonara YE, Du Toit LC, Kumar P, Mabrouk M, Kondiah PPD, Pillay V. A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents. Polymers. 2016; 8(10):359. https://doi.org/10.3390/polym8100359
Chicago/Turabian StyleZardad, Az-Zamakhshariy, Yahya Essop Choonara, Lisa Claire Du Toit, Pradeep Kumar, Mostafa Mabrouk, Pierre Pavan Demarco Kondiah, and Viness Pillay. 2016. "A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents" Polymers 8, no. 10: 359. https://doi.org/10.3390/polym8100359
APA StyleZardad, A. -Z., Choonara, Y. E., Du Toit, L. C., Kumar, P., Mabrouk, M., Kondiah, P. P. D., & Pillay, V. (2016). A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents. Polymers, 8(10), 359. https://doi.org/10.3390/polym8100359