Iron-Mediated Homogeneous ICAR ATRP of Methyl Methacrylate under ppm Level Organometallic Catalyst Iron(III) Acetylacetonate
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. General Procedure for ICAR ATRP of MMA
2.3. Chain Extension of PMMA
2.4. Characterization
3. Results and Discussion
3.1. Effect of Type of Initiator and Solvent on Polymerization of MMA
| Entry | R | T (h) | Conv. (%) | Mn,th f (g/mol) | Mn,GPC (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 a | 200:1:0.02:0.3:1 | 3.5 | 64.0 | 12,800 | 17,000 | 1.18 |
| 2 a | 200:1:0.015:0.3:1 | 3.5 | 54.7 | 10,900 | 15,900 | 1.19 |
| 3 b | 200:1:0.02:0.3:1 | 1.5 | 43.7 | 8800 | 96,900 | 1.56 |
| 4 b | 200:1:0.01:0.3:1 | 1.5 | 43.5 | 8700 | 96,500 | 1.46 |
| 5 c | 200:1:0.02:0.3:1 | 3 | 56.0 | 11,200 | 16,500 | 1.17 |
| 6 d | 200:1:0.02:0.3:1 | 3 | 53.1 | 10,600 | 24,600 | 1.40 |
| 7 e | 200:1:0.02:0.3:1 | 3 | 36.1 | 7200 | 9500 | 1.18 |
3.2. Comparison of Using AIBN and ACHN as the Thermal Initiator
| Entry | R | T (h) | Conv. (%) | Mn,th c (g/mol) | Mn,GPC (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 a | 200:1:0.02:0.3:1 | 2.5 | 40.8 | 8200 | 14,900 | 1.16 |
| 2 a | 200:1:0.01:0.3:1 | 2.5 | 37.6 | 7500 | 17,100 | 1.22 |
| 3 a | 200:1:0.001:0.3:1 | 2 | 58.2 | 11,600 | 24,200 | 1.42 |
| 4 b | 200:1:0.02:0.3:1 | 3.5 | 64.0 | 12,800 | 17,000 | 1.18 |
| 5 b | 200:1:0.015:0.3:1 | 3.5 | 54.7 | 10,900 | 15,900 | 1.19 |
| 6 b | 200:1:0.001:0.3:1 | 3 | 50.1 | 10,000 | 21,100 | 1.27 |
3.3. Effect of Concentration of Iron Catalyst on Polymerization of MMA
| Entry | Catalyst Concentration (ppm) | T (h) | Conv. (%) | Mn,th a (g/mol) | Mn,GPC (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 150 | 2 | 27.4 | 5500 | 10,600 | 1.17 |
| 2 | 150 | 4 | 66.5 | 13,300 | 19,500 | 1.13 |
| 3 | 100 | 2 | 41.0 | 8200 | 15,700 | 1.20 |
| 4 | 100 | 4 | 68.0 | 13,600 | 19,000 | 1.14 |
| 5 | 75 | 2 | 30.6 | 7100 | 11,800 | 1.23 |
| 6 | 75 | 4 | 63.0 | 12,600 | 17,400 | 1.18 |
| 7 | 5 | 3 | 50.1 | 10,000 | 21,140 | 1.27 |
| 8 | 2.5 | 2 | 45.2 | 9000 | 32,200 | 1.39 |
| 9 | 1 | 2 | 45.9 | 9200 | 43,300 | 1.43 |
3.4. Effect of Concentration of ACHN on Polymerization of MMA
| Entry | x | Conv. (%) | Mn,th a (g/mol) | Mn,GPC (g/mol) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | 0.1 | – | – | – | – |
| 2 | 0.5 | 24.4 | 4900 | 10,400 | 1.19 |
| 3 | 1.0 | 48.0 | 9600 | 16,400 | 1.30 |
| 4 | 1.5 | 62.6 | 12,500 | 16,700 | 1.17 |
| 5 | 2.0 | 63.2 | 12,600 | 18,400 | 1.17 |
| 6 | 2.5 | 73.5 | 14,700 | 19,900 | 1.21 |
3.5. Variation of the Target Degree of Polymerization
| Entry | R | Conv.(%) | a Mn,th (g/mol ) | Mn,GPC (g/mol ) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | 300:1:0.02:0.3:1 | 43.9 | 13,200 | 21,500 | 1.15 |
| 2 | 400:1:0.02:0.3:1 | 43.0 | 17,200 | 24,900 | 1.16 |
| 3 | 800:1:0.02:0.3:1 | 39.6 | 31,700 | 41,100 | 1.20 |
| 4 | 1000:1:0.02:0.3:1 | 36.3 | 36,300 | 43,300 | 1.24 |
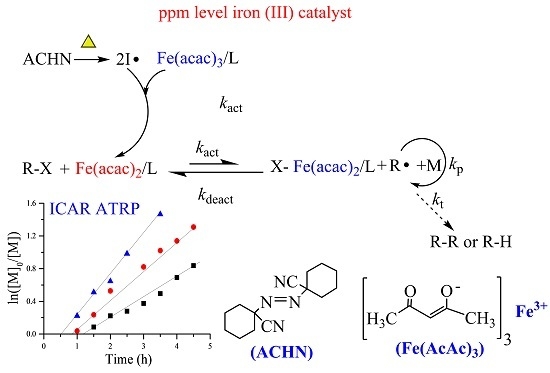
3.6. Polymerization Mechanism and Polymerization Kinetics



3.7. Analysis of Chain-End and Chain Extension


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shipp, D.A. Reversible-deactivation radical polymerizations. Polym. Rev. 2011, 51, 99–103. [Google Scholar] [CrossRef]
- Zhao, J.F.; Wang, W.X.; Bai, L.J.; Zhou, L.L.; Cheng, Z.P.; Zhang, Z.B.; Zhu, X.L. Reversible-deactivation radical polymerization mediated by CuSO4·5H2O: An alternative and promising copper(II)-based catalyst. Polym. Chem. 2012, 3, 3220–3223. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, M.; Zhu, W.; Peng, C.H.; Zhang, Y.; Konkolewicz, D.; Bortoamei, N.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Reversible-deactivation radical polymerization in the presence of metallic copper. Comproportionation–disproportionation equilibria and kinetics. Macromolecules 2013, 46, 3793–3802. [Google Scholar] [CrossRef]
- Peng, C.H.; Yang, T.Y.; Zhao, Y.G.; Fu, X.F. Reversible deactivation radical polymerization mediated by cobalt complexes: Recent progress and perspectives. Org. Biomol. Chem. 2014, 12, 8580–8587. [Google Scholar] [CrossRef] [PubMed]
- Poli, R.; Allan, L.E.; Shaver, M.P. Iron-mediated reversible deactivation controlled radical polymerization. Prog. Polym. Sci. 2014, 39, 1827–1845. [Google Scholar] [CrossRef]
- Feng, C.; Lu, G.L.; Sun, G.; Liu, X.W.; Huang, X.Y. t BCPMA: A new trifunctional acrylic monomer for convenient synthesis of a well-defined amphiphilic graft copolymer by successive RDRP. Polym. Chem. 2014, 5, 6027–6038. [Google Scholar] [CrossRef]
- Zhang, M.M.; Cunningham, M.F.; Hutchinson, R.A. Aqueous copper(0) mediated reversible deactivation radical polymerization of 2-hydroxyethyl acrylate. Polym. Chem. 2015, 6, 6509–6518. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Tazaki, T. A model for living radical polymerization. Makromol. Chem. Rapid Commun. 1982, 3, 133–140. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M. Role of initiator-transfer agent-terminator (iniferter) in radical polymerizations: Polymer design by organic disulfides as iniferters. Makromol. Chem. Rapid Commun. 1982, 3, 127–132. [Google Scholar] [CrossRef]
- Otsu, T. Iniferter concept and living radical polymerization. J. Polym. Sci. Part A 2000, 38, 2121–2136. [Google Scholar] [CrossRef]
- Šebenik, A. Living free-radical block copolymerization using thio-iniferters. Prog. Polym. Sci. 1998, 23, 875–917. [Google Scholar] [CrossRef]
- García-Valdez, O.; Champagne-Hartley, R.; Saldívar-Guerra, E.; Champagne, P.; Cunningham, M.F. Modification of chitosan with polystyrene and poly(n-butyl acrylate) via nitroxide-mediated polymerization and grafting from approach in homogeneous media. Polym. Chem. 2015, 6, 2827–2836. [Google Scholar] [CrossRef]
- Chen, S.; Alves, M.H.; Save, M.; Billon, L. Synthesis of amphiphilic diblock copolymers derived from renewable dextran by nitroxide mediated polymerization: Towards hierarchically structured honeycomb porous films. Polym. Chem. 2014, 5, 5310–5319. [Google Scholar] [CrossRef]
- Cherifi, N.; Issoulie, A.; Khoukh, A.; Benaboura, A.; Save, M.; Derail, C.; Billon, L. Synthetic methodology effect on the microstructure and thermal properties of poly(n-butyl acrylate-co-methyl methacrylate) synthesized by nitroxide mediated polymerization. Polym. Chem. 2011, 2, 1769–1777. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Bertin, D.; Gigmes, D.; Marque, S.R.; Tordo, P. Kinetic subtleties of nitroxide mediated polymerization. Chem. Soc. Rev. 2011, 40, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, R.B. Nitroxide-mediated radical polymerization: Limitations and versatility. Polym. Rev. 2011, 51, 104–137. [Google Scholar] [CrossRef]
- Sciannamea, V.; Jérôme, R.; Detrembleur, C. In-situ nitroxide-mediated radical polymerization (NMP) processes: Their understanding and optimization. Chem. Rev. 2008, 108, 1104–1126. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium (II)/methylaluminum bis (2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/”living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Terashima, T.; Sawamoto, M. Transition metal-catalyzed living radical polymerization: Toward perfection in catalysis and precision polymer synthesis. Chem. Rev. 2009, 109, 4963–5050. [Google Scholar] [CrossRef] [PubMed]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: from process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Activators generated by electron transfer for atom transfer radical polymerization: recent advances in catalyst and polymer chemistry. Polym. Chem. 2012, 3, 2685–2697. [Google Scholar] [CrossRef]
- He, W.W.; Jiang, H.J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Atom transfer radical polymerization of hydrophilic monomers and its applications. Polym. Chem. 2013, 4, 2919–2938. [Google Scholar] [CrossRef]
- Pan, J.L.; Li, Z.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Iron-mediated AGET ATRP of styrene and methyl methacrylate using ascorbic acid sodium salt as reducing agent. Chin. J. Polym. Sci. 2014, 32, 1010–1018. [Google Scholar] [CrossRef]
- Jiang, X.W.; Luo, Y.J.; Li, Z.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Thermoregulated phase transfer catalysis in aqueous/organic biphasic system: facile and highly efficient ATRP catalyst separation and recycling in situ using typical alkyl halide as initiator. Polym. Chem. 2015, 6, 6394–6401. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.P.; Liu, X.H.; Wang, C.P.; Wang, J.F.; Chu, F.X.; Tang, C.B. Integration of renewable cellulose and rosin towards sustainable copolymers by “grafting from” ATRP. Green Chem. 2014, 16, 1854–1864. [Google Scholar] [CrossRef]
- Ouchi, M.; Tokuoka, S.; Sawamoto, M. Halogen donors in metal-catalyzed living radical polymerization: Control of the equilibrium between dormant and active species. Macromolecules 2008, 41, 518–520. [Google Scholar] [CrossRef]
- Pan, J.L.; Zhang, L.F.; Bai, L.J.; Zhang, Z.B.; Chen, H.; Cheng, Z.P.; Zhu, X.L. Atom transfer radical polymerization of methyl methacrylate with a thermo-responsive ligand: Construction of thermoregulated phase-transfer catalysis in an aqueous-organic biphasic system. Polym. Chem. 2013, 4, 2876–2883. [Google Scholar] [CrossRef]
- Zhu, G.H.; Zhang, L.F.; Pan, X.Q.; Zhang, W.; Cheng, Z.P.; Zhu, X.L. Facile soap-free miniemulsion polymerization of methyl methacrylate via reverse atom transfer radical polymerization. Macromol. Rapid Commun. 2012, 33, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.Q.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Recent progress on transition metal catalyst separation and recycling in ATRP. Macromol. Rapid Commun. 2015, 36, 1702–1721. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.J.; Zhang, L.F.; Pan, J.L.; Zhu, J.; Cheng, Z.P.; Zhu, X.L. Developing a synthetic approach with thermoregulated phase-transfer catalysis: facile access to metal-mediated living radical polymerization of methyl methacrylate in aqueous/organic biphasic system. Macromolecules 2013, 46, 2060–2066. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Zhu, X.L.; Zhang, L.F.; Zhou, N.C.; Chen, J.Y. Homogeneous solution reverse atom transfer radical polymerization of methyl methacrylate. J. Macromol. Sci. Pure Appl. Chem. 2003, 40, 371–385. [Google Scholar] [CrossRef]
- Miao, J.; He, W.W.; Zhang, L.F.; Wang, Y.; Cheng, Z.P.; Zhu, X.L. AGET ATRP of water-soluble PEGMA: Fast living radical polymerization mediated by iron catalyst. J. Polym. Sci. Part A 2012, 50, 2194–2200. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Zhu, X.L.; Zhang, L.F.; Zhou, N.C.; Xue, X.R. RATRP of MMA in AIBN/FeCl3/PPh3 initiation system under microwave irradiation. Polym. Bull. 2003, 49, 363–369. [Google Scholar] [CrossRef]
- Jiang, X.W.; Liu, Y.; Ding, M.Q.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. AGET ATRP of methyl methacrylate based on thermoregulated phase transfer catalysis in organic/aqueous biphasic system: Facile and highly efficient in situ catalyst/ligand separation and recycling. Macromol. Chem. Phys. 2015, 216, 1171–1179. [Google Scholar] [CrossRef]
- Ding, M.Q.; Jiang, X.W.; Peng, J.Y.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Diffusion-regulated phase-transfer catalysis for atom transfer radical polymerization of methyl methacrylate in an aqueous/organic biphasic system. Macromol. Rapid Commun. 2015, 36, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Zhang, B.J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Cu(II)-mediated atom transfer radical polymerization of methyl methacrylate via a strategy of thermo-regulated phase-separable catalysis in a liquid/liquid biphasic system: Homogeneous catalysis, facile heterogeneous separation, and recycling. Macromol. Rapid Commun. 2014, 35, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Pan, J.L.; Chen, M.T.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Thermo-regulated phase separable catalysis (TPSC)-based atom transfer radical polymerization in a thermo-regulated ionic liquid. Chem. Commun. 2014, 50, 9266–9269. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Jiang, H.J.; Zhang, L.F.; Wu, Z.Q.; Cheng, Z.P.; Zhu, X.L. AGET ATRP of methyl methacrylate via a bimetallic catalyst. RSC Adv. 2012, 2, 840–847. [Google Scholar] [CrossRef]
- Fujimura, K.; Ouchi, M.; Sawamoto, M. Ferrocene cocatalysis for iron-catalyzed living radical polymerization: Active, robust, and sustainable system under concerted catalysis by two iron complexes. Macromolecules 2015, 48, 4294–4300. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.; Mayadunne, R.T.; Meijs, G.F.; Moad, G.; Rizzardo, E. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Haven, J.J.; Guerrero-Sanchez, C.; Keddie, D.J.; Moad, G.; Thang, S.H.; Schubert, U.S. One pot synthesis of higher order quasi-block copolymer libraries via sequential RAFT polymerization in an automated synthesizer. Polym. Chem. 2014, 5, 5236–5246. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Zhang, Z.B.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. A surfactant-free emulsion RAFT polymerization of methyl methacrylate in a continuous tubular reactor. Polym. Chem. 2015, 6, 1937–1943. [Google Scholar] [CrossRef]
- Shi, P.; Zhou, H.; Gao, C.; Wang, S.; Sun, P.; Zhang, W. Macro-RAFT agent mediated dispersion copolymerization: A small amount of solvophilic co-monomer leads to a great change. Polym. Chem. 2015, 6, 4911–4920. [Google Scholar] [CrossRef]
- Xu, T.C.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. A novel methacrylate with a bisphosphonate group: RAFT polymerization and flame retardant property of the resultant polymers. Polym. Chem. 2015, 6, 2283–2289. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Narain, R. Progress of RAFT based polymers in gene delivery. Prog. Polym. Sci. 2013, 38, 767–790. [Google Scholar] [CrossRef]
- Gregory, A.; Stenzel, M.H. Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature's building blocks. Prog. Polym. Sci. 2012, 37, 38–105. [Google Scholar] [CrossRef]
- Beija, M.; Marty, J.D.; Destarac, M. RAFT/MADIX polymers for the preparation of polymer/inorganic nanohybrids. Prog. Polym. Sci. 2011, 36, 845–886. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Gaynor, S.G.; Müller, A.H. Preparation of hyperbranched polyacrylates by atom transfer radical polymerization. 2. Kinetics and mechanism of chain growth for the self-condensing vinyl polymerization of 2-((2-bromopropionyl) oxy) ethyl acrylate. Macromolecules 1997, 30, 7034–7041. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: From stars to gels. Prog. Polym. Sci. 2009, 34, 317–350. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Monteiro, M.J.; Perrier, S. Dendritic and hyperbranched polymers from macromolecular units: elegant approaches to the synthesis of functional polymers. Macromolecules 2011, 44, 7067–7087. [Google Scholar] [CrossRef]
- Mueller, L.; Jakubowski, W.; Tang, W.; Matyjaszewski, K. Successful chain extension of polyacrylate and polystyrene macroinitiators with methacrylates in an ARGET and ICAR ATRP. Macromolecules 2007, 40, 6464–6472. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Magenau, A.J.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. ICAR ATRP with ppm Cu catalyst in water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, L.F.; Pan, X.Q.; Li, X.H.; Cheng, Z.P.; Zhu, X.L. A highly active homogeneous ICAR ATRP of methyl methacrylate using ppm levels of organocopper catalyst. Polym. Chem. 2013, 4, 3725–3734. [Google Scholar] [CrossRef]
- Zhang, B.J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Fe (iii)-mediated ICAR ATRP in ap-xylene/PEG-200 biphasic system: Facile and highly efficient separation and recycling of an iron catalyst. Polym. Chem. 2015, 6, 6616–6622. [Google Scholar] [CrossRef]
- Okada, S.; Park, S.; Matyjaszewski, K. Initiators for continuous activator regeneration atom transfer radical polymerization of methyl methacrylate and styrene with N-heterocyclic carbene as ligands for Fe-based catalysts. ACS Macro Lett. 2014, 3, 944–947. [Google Scholar] [CrossRef]
- Zhang, L.F.; Miao, J.; Cheng, Z.P.; Zhu, X.L. Iron-mediated ICAR ATRP of styrene and methyl methacrylate in the absence of thermal radical initiator. Macromol. Rapid Commun. 2010, 31, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Pintauer, T.; Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 2008, 37, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.W.; Wu, J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Highly active ppm level organic copper catalyzed photo-induced ICAR ATRP of methyl methacrylate. Macromol. Rapid Commun. 2014, 35, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Plichta, A.; Li, W.; Matyjaszewski, K. ICAR ATRP of styrene and methyl methacrylate with Ru(Cp*)Cl(PPh3)2. Macromolecules 2009, 42, 2330–2332. [Google Scholar] [CrossRef]
- Zhu, G.H.; Zhang, L.F.; Zhang, Z.B.; Zhu, J.; Tu, Y.F.; Cheng, Z.P.; Zhu, X.L. Iron-mediated ICAR ATRP of methyl methacrylate. Macromolecules 2011, 44, 3233–3239. [Google Scholar] [CrossRef]
- Wang, G.X.; Lu, M.; Liu, Y. ICAR ATRP of methyl methacrylate catalyzed by FeCl3·6H2O/succinic acid. J. Appl. Polym. Sci. 2012, 126, 381–386. [Google Scholar] [CrossRef]
- Ando, T.; Kamigaito, M.; Sawamoto, M. Iron(II) chloride complex for living radical polymerization of methyl methacrylate 1. Macromolecules 1997, 30, 4507–4510. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Wei, M.; Xia, J.; Gaynor, S.G. Atom transfer radical polymerization of styrene catalyzed by copper carboxylate complexes. Macromol. Chem. Phys. 1998, 199, 2289–2292. [Google Scholar] [CrossRef]
- Schroeder, H.; Lake, B.R.; Demeshko, S.; Shaver, M.P.; Buback, M. A Synthetic and multispectroscopic speciation analysis of controlled radical polymerization mediated by amine–bis (phenolate)iron complexes. Macromolecules 2015, 48, 4329–4338. [Google Scholar] [CrossRef]
- Nakanishi, S.I.; Kawamura, M.; Kai, H.; Jin, R.H.; Sunada, Y.; Nagashima, H. Well-defined iron complexes as efficient catalysts for “green” atom-transfer radical polymerization of styrene, methyl methacrylate, and butyl acrylate with low catalyst loadings and catalyst recycling. Chem. Eur. J. 2014, 20, 5802–5814. [Google Scholar] [CrossRef] [PubMed]
- Simakova, A.; Mackenzie, M.; Averick, S.E.; Park, S.; Matyjaszewski, K. Bioinspired iron-based catalyst for atom transfer radical polymerization. Angew. Chem. Int. Ed. 2013, 52, 12148–12151. [Google Scholar] [CrossRef] [PubMed]
- He, W.W.; Zhang, L.F.; Miao, J.; Cheng, Z.P.; Zhu, X.L. Facile iron-mediated AGET ATRP for water-soluble poly(ethylene glycol) monomethyl ether methacrylate in water. Macromol. Rapid Commun. 2012, 33, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.J.; Zhang, L.F.; Zhu, J.; Cheng, Z.P.; Zhu, X.L. Iron(III)-mediated AGET ATRP of styrene using tris(3,6-dioxaheptyl) amine as a ligand. J. Polym. Sci. Part A 2009, 47, 2002–2008. [Google Scholar] [CrossRef]
- Zhang, L.F.; Cheng, Z.P.; Lü, Y.T.; Zhu, X.L. A highly active iron-based catalyst system for the AGET ATRP of styrene. Macromol. Rapid Commun. 2009, 30, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Sen, A. Electron-transfer-induced iron-based atom transfer radical polymerization of styrene derivatives and copolymerization of styrene and methyl methacrylate. Macromolecules 2008, 41, 4514–4518. [Google Scholar] [CrossRef]
- Mohammad Rabea, A.; Zhu, S. Controlled radical polymerization at high conversion: Bulk ICAR ATRP of methyl methacrylate. Ind. Eng. Chem. Res. 2014, 53, 3472–3477. [Google Scholar] [CrossRef]
- Schroeder, H.; Matyjaszewski, K.; Buback, M. Kinetics of Fe-mediated ATRP with triarylphosphines. Macromolecules 2015, 48, 4431–4437. [Google Scholar] [CrossRef]
- Bai, L.J.; Wang, W.X.; Chen, H.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Facile iron(iii)-mediated ATRP of MMA with phosphorus-containing ligands in the absence of any additional initiators. RSC Adv. 2015, 5, 62577–62584. [Google Scholar] [CrossRef]
- Xue, Z.G.; He, D.; Xie, X.L. Iron-catalyzed atom transfer radical polymerization. Polym. Chem. 2015, 6, 1660–1687. [Google Scholar] [CrossRef]
- Khan, M.Y.; Chen, X.; Lee, S.W.; Noh, S.K. Development of new atom transfer radical polymerization system by iron(III)-metal salts without using any external initiator and reducing agent. Macromol. Rapid Commun. 2013, 34, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Ouchi, M.; Sawamoto, M. Phosphine–ligand decoration toward active and robust iron catalysts in LRP. Macromolecules 2013, 46, 3342–3349. [Google Scholar] [CrossRef]
- Aoshima, H.; Satoh, K.; Umemura, T.; Kamigaito, M. A simple combination of higher-oxidation-state FeX3 and phosphine or amine ligand for living radical polymerization of styrene, methacrylate, and acrylate. Polym. Chem. 2013, 4, 3554–3562. [Google Scholar] [CrossRef]
- Wang, Y.; Kwak, Y.; Matyjaszewski, K. Enhanced activity of ATRP Fe catalysts with phosphines containing electron donating Groups. Macromolecules 2012, 45, 5911–5915. [Google Scholar] [CrossRef]
- Bai, L.J.; Zhang, L.F.; Zhang, Z.B.; Zhu, J.; Zhou, N.C.; Cheng, Z.P.; Zhu, X.L. Alumina additives for fast iron-mediated AGET ATRP of MMA using onium salt as ligand. J. Polym. Sci., Part A 2011, 49, 3970–3979. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Matyjaszewski, K. ATRP of methyl acrylate with metallic zinc, magnesium, and iron as reducing agents and supplemental activators. Macromolecules 2011, 44, 683–685. [Google Scholar] [CrossRef]
- Deng, Z.J.; Guo, J.N.; Qiu, L.H.; Yuan, C.; Zhou, Y.X.; Yan, F. Iron-mediated AGET ATRP of MMA with sulfosalicylic acid as a ligand. J. Polym. Sci., Part A 2013, 51, 664–671. [Google Scholar] [CrossRef]
- Hou, C.; Qu, R.J.; Liu, J.S.; Guo, Z.L.; Wang, C.H.; Ji, C.N.; Sun, C.M.; Wang, C.G. Reverse ATRP of acrylonitrile with diethyl 2,3-dicyano-2,3-diphenyl succinate/FeCl3/iminodiacetic acid. Polymer 2006, 47, 1505–1510. [Google Scholar] [CrossRef]
- Zhu, S.M.; Yan, D.Y. New ligands for atom-transfer radical polymerization. Macromol. Rapid Commun. 2000, 21, 1209–1213. [Google Scholar] [CrossRef]
- Zhu, S.M.; Yan, D.Y. Atom transfer radical polymerization of methyl methacrylate catalyzed by ironII chloride/isophthalic acid system. Macromolecules 2000, 33, 8233–8238. [Google Scholar] [CrossRef]
- Ding, M.Q.; Jiang, X.W.; Peng, J.Y.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. An atom transfer radical polymerization system: Catalyzed by an iron catalyst in PEG-400. Green Chem. 2015, 17, 271–278. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.C.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Bulk AGET ATRP of methyl methacrylate using iron(iii) acetylacetonate as a catalyst. Polym. Chem. 2014, 5, 6804–6810. [Google Scholar] [CrossRef]
- Mukumoto, K.; Wang, Y.; Matyjaszewski, K. Iron-based ICAR ATRP of styrene with ppm amounts of FeIIIBr3 and 1,1′-azobis (cyclohexanecarbonitrile). ACS Macro Lett. 2012, 1, 599–602. [Google Scholar] [CrossRef]
- Jakubowski, W.; Matyjaszewski, K. Activators regenerated by electron transfer for atom-transfer radical polymerization of (meth)acrylates and related block copolymers. Angew. Chem. 2006, 118, 4594–4598. [Google Scholar] [CrossRef]
- Bai, L.J.; Zhang, L.F.; Zhang, Z.B.; Tu, Y.F.; Zhou, N.C.; Cheng, Z.P.; Zhu, X.L. Iron-mediated AGET ATRP of styrene in the presence of catalytic amounts of base. Macromolecules 2010, 43, 9283–9290. [Google Scholar] [CrossRef]
- Jiang, H.J.; Zhang, L.F.; Pan, J.L.; Jiang, X.W.; Cheng, Z.P.; Zhu, X.L. Iron-mediated AGET ATRP of methyl methacrylate using metal wire as reducing agent. J. Polym. Sci. Part A 2012, 50, 2244–2253. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Jiang, X.; Zhang, L.; Cheng, Z.; Zhu, X. Iron-Mediated Homogeneous ICAR ATRP of Methyl Methacrylate under ppm Level Organometallic Catalyst Iron(III) Acetylacetonate. Polymers 2016, 8, 29. https://doi.org/10.3390/polym8020029
Wu J, Jiang X, Zhang L, Cheng Z, Zhu X. Iron-Mediated Homogeneous ICAR ATRP of Methyl Methacrylate under ppm Level Organometallic Catalyst Iron(III) Acetylacetonate. Polymers. 2016; 8(2):29. https://doi.org/10.3390/polym8020029
Chicago/Turabian StyleWu, Jian, Xiaowu Jiang, Lifen Zhang, Zhenping Cheng, and Xiulin Zhu. 2016. "Iron-Mediated Homogeneous ICAR ATRP of Methyl Methacrylate under ppm Level Organometallic Catalyst Iron(III) Acetylacetonate" Polymers 8, no. 2: 29. https://doi.org/10.3390/polym8020029
APA StyleWu, J., Jiang, X., Zhang, L., Cheng, Z., & Zhu, X. (2016). Iron-Mediated Homogeneous ICAR ATRP of Methyl Methacrylate under ppm Level Organometallic Catalyst Iron(III) Acetylacetonate. Polymers, 8(2), 29. https://doi.org/10.3390/polym8020029