Reversible Self-Assembly of Backbone-Thermoresponsive Long Chain Hyperbranched Poly(N-Isopropyl Acrylamide)
Abstract
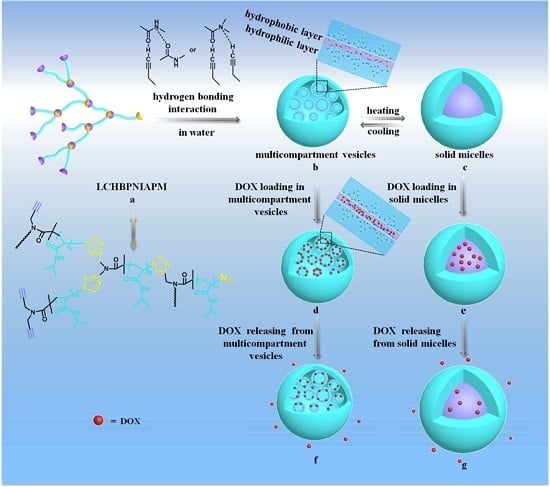
:1. Introduction
2. Materials

3. Characterization Methods
3.1. Polymer Solution Characterization
3.2. Polymer Solution Properties
3.3. Drug Loading and Vitro Release
4. Results and Discussion

| Temperature (°C) | Dav,TEM (nm) a | Dz (nm) b | PDI c | Zeta (mV) d | Rg/Rh e |
|---|---|---|---|---|---|
| 20 | 194 ± 0.6 | 264 ± 0.4 | 0.504 | −0.6 ± 0.3 | 0.96 |
| 45 heating | 345 ± 0.4 | 469 ± 0.5 | 0.248 | −14.8 ± 0.4 | - |
| 65 heating | 540 ± 0.4 | 637 ± 0.3 | - | −23.2 ± 1.2 | - |
| 45 cooling | 301 ± 0.5 | 471 ± 0.4 | 0.183 | −26.2 ± 0.7 | - |
| 20 cooling | 222 ± 0.6 | 258 ± 0.6 | 0.275 | −3.6 ± 0.5 | 1.07 |






5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures—Synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yan, D.Y. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Yan, D.Y. Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: Progress, characteristics and perspectives. Chem. Commun. 2009, 6, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, J.Y.; Wu, J.L.; Li, G.L.; Wang, R.B.; Su, Y.; He, P.; Zhu, X.Y.; Yan, D.Y.; Zhu, B.S. Synthesis, characterization, and in vitro evaluation of long-chain hyperbranched poly(ethylene glycol) as drug carrier. Bioconj. Chem. 2010, 21, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Monteiro, J.M.; Perrier, S. Dendritic and hyperbranched polymers from macromolecular units: Elegant approaches to the synthesis of functional polymers. Macromolecules 2011, 44, 7076–7087. [Google Scholar] [CrossRef]
- Hutchings, L.R.; Roberts-Bleming, S.J. DendriMacs. Well-defined dendritically branched polymers synthesized by an iterative convergent strategy involving the coupling reaction of AB2 macromonomers. Macromolecules 2006, 39, 2144–2152. [Google Scholar] [CrossRef] [Green Version]
- Konkolewicz, D.; Gray-Weale, A.; Perrier, S. Hyperbranched polymers by thiol-yne chemistry: From small molecules to functional polymers. J. Am. Chem. Soc. 2009, 131, 18075–18077. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, W.D.; Li, L.W.; Jiang, W.X.; Tao, J.; Yang, J.; Chen, L.; Ge, X.S.; Chen, S.Q. Controlling the formation of long-subchain hyperbranched polystyrene from seesaw-type AB2 macromonomers: Solvent polarity and solubility. J. Polym. Sci. A Polym. Chem. 2012, 50, 3214–3224. [Google Scholar] [CrossRef]
- Hutchings, L.R.; Dodds, J.M.; Roberts-Bleming, S.J. HyperMacs. Long chain branched analogues of hyperbranched polymers prepared by the polycondensation of AB2 macromonomers. Macromol. Symp. 2006, 240, 56–67. [Google Scholar] [CrossRef]
- Xie, C.; Ju, Z.H.; Zhang, C.; Yang, Y.L.; He, J.P. Dendritic block and dendritic brush copolymers through anionic macroinimer approach. Macromolecules 2013, 46, 1437–1446. [Google Scholar] [CrossRef]
- Zheng, Y.; Thurecht, K.J.; Wang, W.X. Polysiloxanes Polymers with hyperbranched structure and multivinyl functionality. J. Polym. Sci. A Polym. Chem. 2012, 50, 629–637. [Google Scholar] [CrossRef]
- Jiang, W.X.; He, W.D.; He, C. Synthesis of long-subchain hyperbranched PCL through SCV-ATRP of macroinimers with (meth)acrlate group. J. Polym. Sci. A Polym. Chem. 2012, 50, 3475–3480. [Google Scholar] [CrossRef]
- Malmstrom, E.; Johansson, M.; Hult, A. The effect of terminal alkyl chains on hyperbranched polyesters based on 2,2-bis (hydroxymethyl) propionic acid. Macromol. Chem. Phys. 1996, 197, 3199–3207. [Google Scholar] [CrossRef]
- Li, L.W.; Zhou, J.F.; Wu, C. Intrachain folding and interchain association of hyperbranched chains with long uniform subchains made of amphiphilic diblock copolymers. Macromolecules 2012, 45, 9391–9399. [Google Scholar] [CrossRef]
- Jikei, M.; Suzuki, M.; Itoh, K.; Matsumoto, K.; Saito, Y.; Kawaguchi, S. Synthesis of hyperbranched poly(l-lactide)s by self-polycondensation of AB2 macromonomers and their structural characterization by light scattering measurements. Macromolecules 2012, 45, 8237–8244. [Google Scholar] [CrossRef]
- Mishra, K.; Joy, A. Dual functionalized telechelic block copolymers with reproducible block sizes prepared by microwave assisted RAFT polymerization. Polymer 2015, 66, 110–121. [Google Scholar] [CrossRef]
- Hutchings, L.R.; Dodds, J.M.; Roberts-Bleming, S.J. HyperMacs: Highly branched polymers prepared by the polycondensation of AB2 macromonomers, synthesis and characterization. Macromolecules 2005, 38, 5970–5980. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click-Chemie: Diverse chemische Funktionalität mit einer Handvoll guter Reaktionen. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. “Click” chemistry in polymer and materials science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Whittaker, M.R.; Urbani, C.N.; Monteiro, M.J. Synthesis of 3-miktoarm stars and 1st generation mikto dendritic copolymers by “living” radical polymerization and “click” chemistry. J. Am. Chem. Soc. 2006, 128, 11360–11361. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.F.; Matyjaszewski, K. Synthesis of star polymers by a combination of ATRP and the “Click” coupling method. Macromolecules 2006, 39, 4960–4965. [Google Scholar] [CrossRef]
- Englert, B.C.; Bakbak, S.; Bunz, U.H.F. Click chemistry as a powerful tool for the construction of functional Poly(p-phenyleneethynylene)s: Comparison of pre- and post-functionalization schemes. Macromolecules 2005, 38, 5868–5877. [Google Scholar] [CrossRef]
- Li, Z.K.; Sun, M.; Qiao, H.M.; Pan, C.Y. Synthesis and characterization of hyperbranched polystyrene via click reaction of AB2 macromonomer. J. Polym. Sci. A Polym. Chem. 2010, 48, 454–462. [Google Scholar]
- Li, L.W.; He, C.; He, W.; Wu, C. Formation kinetics and scaling of “defect-free” hyperbranched polystyrene chains with uniform subchains prepared from seesaw-type macromonomers. Macromolecules 2011, 44, 8195–8206. [Google Scholar] [CrossRef]
- He, C.; Li, L.W.; He, W.D.; Jiang, W.X.; Wu, C. “Click” long seesaw-type A~~B~~A Chains together into huge defect-free hyperbranched polymer chains with uniform subchains. Macromolecules 2011, 44, 6233–6236. [Google Scholar] [CrossRef]
- Cao, K.; Li, Y.; Lu, Z.Q.; Wu, S.L.; Chen, Z.H.; Yao, Z.; Huang, Z.M. Preparation and characterization of high melt strength polypropylene with long chain branched structure by the reactive extrusion process. J. Appl. Polym. Sci. 2011, 121, 3384–3392. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, Z.Q.; Zhao, X.; Qu, B.W.; Shen, Z.C.; Cao, K. Synthesis and characterization of high-density polypropylene-grafted polyethylene via a macromolecular reaction and its rheological behavior. J. Appl. Polym. Sci. 2009, 111, 2553–2561. [Google Scholar] [CrossRef]
- Dodds, J.M.; Luca, E.D.; Hutchings, L.R.; Clarke, N. Rheological properties of HyperMacs-long-chain branched analogues of hyperbranched polymers. J. Appl. Polym. Sci. B Polym. Phys. 2007, 45, 2762–2769. [Google Scholar] [CrossRef]
- Sugimoto, M.; Koizumi, T.; Taniguchi, T.; Koyama, K.; Saito, K.; Nonokawa, D.; Morita, T. Melt rheology of hyperbranched-polystyrene synthesized with multisite macromonomer. J. Appl. Polym. Sci. B Polym. Phys. 2009, 47, 2226–2237. [Google Scholar] [CrossRef]
- Clarke, N.; Luca, E.D.; Dodds, J.M.; Kimani, S.M.; Hutchings, L.R. HyperMacs—Long chain hyperbranched polymers: A dramatically improved synthesis and qualitative rheological analysis. Eur. Polym. J. 2008, 44, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.Y.; An, L.J.; Wang, Z.G. Intrinsic viscosity of polymers: General theory based on a partially permeable sphere model. Macromolecules 2013, 46, 5731–5740. [Google Scholar] [CrossRef]
- Wang, L.Y.; Jing, X.B.; Cheng, H.B.; Hu, X.L.; Yang, L.X.; Huang, Y.B. Blends of linear and long-chain branched poly(l-lactide)s with high melt strength and fast crystallization rate. Ind. Eng. Chem. Res. 2012, 51, 10088–10099. [Google Scholar] [CrossRef]
- Li, L.W.; Lu, Y.Y.; An, L.J.; Wu, C. Experimental and theoretical studies of scaling of sizes and intrinsic viscosity of hyperbranched chains in good solvents. J. Chem. Phys. 2013, 138, 114908. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Dong, C.M. Synthesis of hyperbranched polypeptide and PEO block copolymer by consecutive thiol-yne chemistry. Biomacromolecules 2013, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jin, B.K.; He, W.D.; Ge, X.S.; Tao, J.; Yang, J.; Chen, S.Q. Solvent replacement to thermo-responsive nanoparticles from long-subchain hyperbranched PSt grafted with PNIPAM for encapsulation. J. Polym. Sci. A Polym. Chem. 2013, 51, 2142–2149. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tang, L.Y.; Li, Y.; Wang, J. Thermoresponsive block copolymers of poly(ethylene glycol) and polyphosphoester: Thermo-induced self-assembly, biocompatibility, and hydrolytic degradation. Biomacromolecules 2009, 10, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Tian, W.; Zhu, Y.Q.; Bai, Y.; Yan, H.X.; Du, J.Z. How does a tiny terminal alkynyl end group drive fully hydrophilic homopolymers to self-assemble into vesicles and flower-like complex particles? Polym. Chem. 2014, 5, 5077–5088. [Google Scholar] [CrossRef]
- Fan, W.W.; Fan, X.D.; Tian, W.; Zhang, X.; Wang, G.; Zhang, W.B.; Bai, Y.; Zhu, X.Z. Phase transition dynamics and mechanism for backbone-thermoresponsive hyperbranched polyethers. Polym. Chem. 2014, 5, 4022–4031. [Google Scholar] [CrossRef]
- Duan, H.W.; Chen, D.Y.; Jiang, M.; Gan, W.J.; Li, S.J.; Wang, M.; Gong, J. Self-assembly of unlike homopolymers into hollow spheres in nonselective solvent. J. Am. Chem. Soc. 2001, 123, 12097–12098. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.E.; Eisenberg, A. Kinetics and mechanisms of the sphere-to-rod and rod-to-sphere transitions in the ternary system PS310-b-PAA52/dioxane/water. Langmuir 2001, 17, 6705–6714. [Google Scholar] [CrossRef]
- Chen, L.; Shen, H.W.; Eisenberg, A. Kinetics and mechanism of the rod-to-vesicle transition of block copolymer aggregates in dilute solution. J. Phys. Chem. B 1999, 103, 9488–9497. [Google Scholar] [CrossRef]
- Liu, J.Y.; Huang, W.; Pang, Y.; Huang, P.; Zhu, X.Y.; Zhou, Y.F.; Yan, D.Y. Molecular self-assembly of a homopolymer: An alternative to fabricate drug-delivery platforms for cancer therapy. Angew. Chem. Int. Ed. 2011, 50, 9162–9166. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Song, L.; Wang, D.L.; Qiu, F.; Yan, D.Y.; Zhu, B.S.; Zhu, X.Y. Synthesis and self-assembly of nonamphiphilic hyperbranched polyoximes. Soft Matter 2012, 8, 10017–10025. [Google Scholar] [CrossRef]
- Liu, J.Y.; Pang, Y.; Chen, J.; Huang, P.; Huang, W.; Zhu, X.Y.; Yan, D.Y. Hyperbranched polydiselenide as a self assembling broad spectrum anticancer agent. Biomaterials 2012, 33, 7765–7774. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.M.; Wang, Y.M.; Wang, Y.P.; Wang, D.L.; Deng, H.P.; Zhuang, Y.Y.; Yan, D.Y.; Zhu, B.S.; Zhu, X.Y. Backbone-thermoresponsive hyperbranched polyglycerol by random copolymerization of glycidol and 3-methyl-3-(hydroxymethyl)oxetane. Macromol. Chem. Phys. 2011, 212, 1056–1062. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Yan, D.Y.; Dong, W.Y.; Tian, Y. Temperature-responsive phase transition of polymer vesicles: Real-time morphology observation and molecular mechanism. J. Phys. Chem. B 2007, 111, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Sun, S.T.; Wu, P.Y. Thermodynamics of hyperbranched poly(ethylenimine) with isobutyramide residues during phase transition: An insight into the molecular mechanism. J. Phys. Chem. B 2011, 115, 8832–8844. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.T.; Zhang, W.D.; Zhang, W.; Wu, P.Y.; Zhu, X.L. Dynamic self-aggregation behavior of a PNIPAM-based nonlinear multihydrophilic block copolymer revealed by two-dimensional correlation spectroscopy. Soft Matter 2012, 8, 3980–3987. [Google Scholar] [CrossRef]
- Sun, S.T.; Wu, P.Y.; Zhang, W.D.; Zhang, W.; Zhu, X.L. Effect of structural constraint on dynamic self-assembly behavior of PNIPAM-based nonlinear multihydrophilic block copolymers. Soft Matter 2013, 9, 1807–1816. [Google Scholar] [CrossRef]
- Naha, P.C.; Bhattacharya, K.; Tenuta, T.; Dawsonc, A.K.; Lynch, I.; Gracia, A.; Lyng, M.F.; Byrne, J.H. Intracellular localisation, geno- and cytotoxic response of polyN-isopropylacrylamide (PNIPAM) nanoparticles to human keratinocyte (HaCaT) and colon cells (SW 480). Toxicol. Lett. 2010, 198, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Burdukova, E.; Li, H.; Bradshaw, D.; Franks, G. Poly(N-isopropylacrylamide) (PNIPAM) as a flotation collector: Effect of temperature and molecular weight. Miner. Eng. 2010, 23, 921–927. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Liu, L.; Du, J.Z. Probing into homopolymer self-assembly: How does hydrogen bonding influence morphology? Macromolecules 2013, 46, 194–203. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Huang, W.; Liu, J.Y.; Zhu, X.Y.; Yan, D.Y. Self-assembly of hyperbranched polymers and its biomedical applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, I.; Zhong, X.F.; Eisenberg, A. Critical micellization phenomena in block polyelectrolyte solutions. Macromolecules 1993, 26, 7339–7352. [Google Scholar] [CrossRef]
- Ohashi, H.; Hiraoka, Y.; Yamaguchi, T. An autonomous phase transition-complexation/decomplexation polymer system with a molecular recognition property. Macromolecules 2006, 39, 2614–2620. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhong, Y.B.; Nan, J.K.; Tian, W. Star polymers with both temperature sensitivity and inclusion functionalities. Macromolecules 2010, 43, 10221–10230. [Google Scholar] [CrossRef]
- Karjalainen, E.; Chenna, N.; Laurinmäki, P.; Butcher, S.J.; Tenhu, H. Diblock copolymers consisting of a polymerized ionic liquid and poly(N-isopropylacrylamide). Effects of PNIPAM block length and counter ion on self-assembling and thermal properties. Polym. Chem. 2013, 4, 1014–1024. [Google Scholar] [CrossRef]
- Tian, W.; Wei, X.Y.; Liu, Y.Y.; Fan, X.D. A branching point thermo and pH dual-responsive hyperbranched polymer based on poly (N-vinylcaprolactam) and poly (N,N-diethyl aminoethyl methacrylate). Polym. Chem. 2013, 4, 2850–2863. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.; Zou, K.D.; Chen, J.; Du, J.Z. Homopolymer vesicles with a gradient bilayer membrane as drug carriers. Chem. Commun. 2013, 49, 11521–11523. [Google Scholar] [CrossRef]
- Nie, S.F.; Hsiao, W.; Pan, W.S.; Yang, Z.J. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2011, 48, 139–157. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-T.; Tian, W.; Song, Y.-L.; Bai, Y.; Wei, P.-L.; Yao, H.; Yan, H.-X. Reversible Self-Assembly of Backbone-Thermoresponsive Long Chain Hyperbranched Poly(N-Isopropyl Acrylamide). Polymers 2016, 8, 33. https://doi.org/10.3390/polym8020033
Liu T-T, Tian W, Song Y-L, Bai Y, Wei P-L, Yao H, Yan H-X. Reversible Self-Assembly of Backbone-Thermoresponsive Long Chain Hyperbranched Poly(N-Isopropyl Acrylamide). Polymers. 2016; 8(2):33. https://doi.org/10.3390/polym8020033
Chicago/Turabian StyleLiu, Ting-Ting, Wei Tian, Yan-Li Song, Yang Bai, Peng-Li Wei, Hao Yao, and Hong-Xia Yan. 2016. "Reversible Self-Assembly of Backbone-Thermoresponsive Long Chain Hyperbranched Poly(N-Isopropyl Acrylamide)" Polymers 8, no. 2: 33. https://doi.org/10.3390/polym8020033
APA StyleLiu, T. -T., Tian, W., Song, Y. -L., Bai, Y., Wei, P. -L., Yao, H., & Yan, H. -X. (2016). Reversible Self-Assembly of Backbone-Thermoresponsive Long Chain Hyperbranched Poly(N-Isopropyl Acrylamide). Polymers, 8(2), 33. https://doi.org/10.3390/polym8020033