1. Introduction
Polyaniline (PANI) is one of the most intriguing conjugated polymers because of the simple and convenient method required to synthesize it, and its tunable conductivity upon doping [
1,
2,
3]. In the present study, we tune optical absorption by the irradiation of Ultra-Violet (UV) light. The irradiation of UV light induces dedoping of PANI. Radical trap reagent aids the dedoping. This phenomenon can be referred to as a new type photochromism.
2. Materials and Techniques
Aniline (Wako, Osaka, Japan) was purified by distillation prior to use. The (−)-Camphor sulfonic acid (Tokyo Chemical Industry (TCI), Tokyo, Japan), sulfuric acid (TCI) and ammonium peroxodisulfate (APS, Kanto Chemical, Tokyo, Japan) were used as received.
Ultra violet and visible (UV-vis) spectroscopy was recorded on a V-630 spectrophotometer (JASCO, Tokyo, Japan). UV light (λ = 375 nm, 0.3 mW/cm2) was irradiated to the PANI. Scanning electron microscope (SEM) observations were carried out with a JSM-7000F (JEOL, Tokyo, Japan) and JED-2200 (JEOL, Tokyo, Japan).
3. Synthesis
Polyaniline doped with (−)-camphor sulfonic acid (PANI/(−)-CSA)
PANI films on indium-tin-oxide (ITO)-coated glass were prepared with the electrochemical polymerization method. An electrolyte was prepared by addition of the aniline (20 mg), (−)-Camphor sulfonic acid (CSA) distilled water (1 mL). Before polymerization, aniline-CSA salt is formed. Then, polymerization was performed in a cell consisting of a sandwiched ITO electrode (cell gap = 0.2 mm) by applying a constant direct-current (DC) voltage of 3.0 V (
Scheme 1a). This method we developed is referred to as sandwich cell polymerization [
4]. The resultant film was deposited on the anode side of an indium-tin-oxide (ITO)-coated glass electrode (
Scheme 1b). The film was washed with distilled water and methanol to remove low-molecular-weight fractions. The chemical formula of this reaction for PANI bearing CSA via static bond (PANI/(−)-CSA) thus prepared is shown in
Scheme 1c.
Scheme 1.
Preparation of PANI/(−)-CSA. (a) Electrochemical polymerization with the two-electrode method; (b) Resultant PANI on ITO; (c) Chemical formula for PANI.
Scheme 1.
Preparation of PANI/(−)-CSA. (a) Electrochemical polymerization with the two-electrode method; (b) Resultant PANI on ITO; (c) Chemical formula for PANI.
4. Optical Spectroscopy
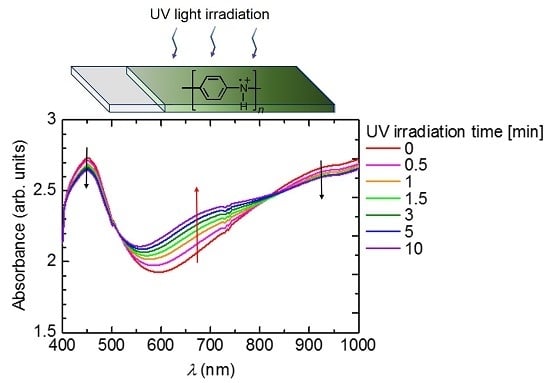
Figure 1 shows the change in optical absorption with the irradiation of UV light. Absorptions at long wavelengths (Vis-NIR range) have doping bands (polarons and bipolarons). The polymer before irradiation of UV light is doped with (−)-CSA as a substituent. Sequential irradiation of UV light induces dedoping, resulting in the long wavelength absorption band being decreased and the absorption band at around 700 nm being increased. Isosbestic points at 500 and 800 nm are observed. This result indicates that the PANI has plural phases, such as emeraldine salt (before irradiation of the UV) and emeraldine base (after irradiation of the UV).
Figure 2 shows the change in the optical absorption of the PANI in the presence of a radical trap reagent (0.01 M dispersion in water) with irradiation of UV light. The
Figure 2 (inset) shows the chemical structure of 4-bromo-2,6-di-
tertbutyl phenol. Before irradiation of UV light, the PANI shows a doping band in the Vis-NIR range. On the other hand, irradiation of UV light changes the spectral form. The new absorption band was observed at 820 nm. This is due to the formation of the emeraldine base state of the main chain. Thus, light-induced dedoping from emeraldine salt to emeraldine base remarkably occurs. At the present stage, the mechanism of dedoping of the CSA-doped polyaniline is unclear. However, the radical trap agent drives the dedoping of the PANI under UV light.
Figure 1.
UV-vis absorption spectra of PANI/(−)-CSA under UV light irradiation.
Figure 1.
UV-vis absorption spectra of PANI/(−)-CSA under UV light irradiation.
Figure 2.
UV-vis absorption spectra of the PANI (0.01 M dispersion in the water) in the presence of 4-bromo-2,6-di-tertbutyl phenol as a radical trap regent under UV light irradiation. Inset shows chemical structure of 4-bromo-2,6-di-tertbutyl phenol.
Figure 2.
UV-vis absorption spectra of the PANI (0.01 M dispersion in the water) in the presence of 4-bromo-2,6-di-tertbutyl phenol as a radical trap regent under UV light irradiation. Inset shows chemical structure of 4-bromo-2,6-di-tertbutyl phenol.
5. Morphology
Scanning electron microscope (SEM) images for the film are shown in
Figure 3. PANI doped with CSA shows a network structure [
5]. The magnification image shows that the electrochemical polymerization produces the anastomosis fibril structure of the PANI.
Figure 3.
Scanning electron microscope (SEM) images of PANI/(−)-CSA. CSA = camphor sulfonic acid. (a) Low magnification; (b) High magnification.
Figure 3.
Scanning electron microscope (SEM) images of PANI/(−)-CSA. CSA = camphor sulfonic acid. (a) Low magnification; (b) High magnification.
6. Conclusions
PANI/(−)-CSA shows photo-induced dedoping at the molecular level. This reaction is irreversible. A radical trap agent accelerates the dedoping. This result demonstrates that the radical trap agent provides electrons to the doped PANI with the aid of UV light irradiation. Although photo-induced doping and photo-conduction are well known phenomena, UV-induced dedoping (color change) is a first example in the research field of conducting polymers.
Acknowledgments
We would like to thank Chemical Analysis Division Research Facility Center for Science and Technology of University of Tsukuba. This work was supported by the National Institute for Materials Science (NIMS) microstructural characterization platform for obtaining SEM images. We thank Takeguchi, Station Director, Transmission Electron Microscopy Station. the National Institute for Materials Science (NIMS).
Author contributions
Yuki Kaitsuka contributed to the experimental measurements. Hiromasa Goto supervised the whole procedure and also contributed to the analysis of the results and the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers. Angew. Chem. Int. Ed. 2001, 40, 2581–2590. [Google Scholar] [CrossRef]
- Yuan, G.; Kuramoto, N. Synthesis of helical polyanilines using chondroitin sulfate as a molecular template Macromol. Chem. Phys. 2004, 205, 1744–1751. [Google Scholar]
- Epstein, A.J.; Ginder, J.M.; Zuo, F.; Bigelow, R.W.; Woo, H.-S.; Tanner, D.B.; Richter, A.F.; Huang, W.-S.; MacDiarmid, A.G. Insulator-to-metal transition in polyaniline. Synth. Met. 1987, 18, 303–309. [Google Scholar] [CrossRef]
- Goto, H. Doping-dedoping-driven optic effect of π-conjugated polymers prepared in cholesteric-liquid-crystal electrolytes. Phys. Rev. Lett. 2007, 98, 253901. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Chougule, M.A.; Pawar, S.G.; Sen, S.; Patil, V.B. Effect of camphor sulfonic acid doping on structural, morphological, optical and electrical transport properties on polyaniline-ZnO nanocomposites. Soft Nanosci. Lett. 2012, 2, 46–53. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).