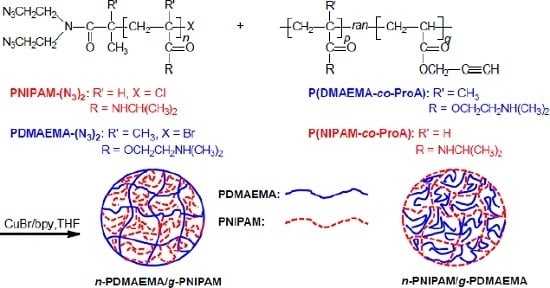
Comb-Type Grafted Hydrogels of PNIPAM and PDMAEMA with Reversed Network-Graft Architectures from Controlled Radical Polymerizations
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Macro-Cross-Linkers of PDMAEMA-(N3)2 and PNIPAM-(N3)2
2.3. Preparation of Alkynyl-Pending Copolymers via RAFT Copolymerization
2.4. Synthesis of Network-Graft Hydrogels through Click Chemistry
2.5. Characterizations of Different Polymers
2.6. Swelling and De-Swelling of Network-Graft Hydrogels
2.7. Drug Loading and Release of Ceftriaxone Sodium by Network-Graft Hydrogels
3. Results and Discussion
3.1. Synthesis of Macro-Cross-Linkers with Di-Azido Groups at One Chain-End



| Polymer | Mn,NMR | Mn,GPC | Mn/Mw by GPC |
|---|---|---|---|
| PDMAEMA40–(N3)2 | 6,400 | 6,850 | 1.13 |
| PDMAEMA60–(N3)2 | 9,600 | 10,100 | 1.15 |
| PDMAEMA120–(N3)2 | 19,000 | 21,350 | 1.20 |
| PNIPAM60–(N3)2 | 7,100 | 7,300 | 1.12 |
| PNIPAM100–(N3)2 | 12,400 | 14,500 | 1.18 |
3.2. Preparation of Alkynyl-Pending Copolymers of P(NIPAM-co-ProA) and P(DMAEMA-co-ProA)

| Polymer | p/q (mole) | Mn,NMR | Mn,GPC | Mn/Mw (GPC) |
|---|---|---|---|---|
| P(NIPAM100-co-ProA5) | 100:5 | 12,100 | 13,400 | 1.24 |
| P(NIPAM100-co-ProA10) | 100:10 | 12,600 | 15,200 | 1.23 |
| PNIPAM100-co-ProA15) | 100:15 | 13,100 | 16,800 | 1.29 |
| P(DMAEMA100-co-ProA5) | 100:5 | 32,100 | 35,700 | 1.44 |
| P(DMAEMA100-co-ProA10) | 100:10 | 33,800 | 36,200 | 1.52 |
| P(DMAEMA100-co-ProA15) | 100:15 | 35,200 | 34,700 | 1.42 |
3.3. Synthesis of pH- and Thermo-Responsive Network-Graft Hydrogels with Reversed Architectures

3.4. Swelling/De-Swelling Behaviors of Network-Graft Hydrogels Dependent on Temperature Change

3.5. pH-Dependent on Swelling/De-Swelling Behaviors of Network-Graft Hydrogels


| Hydrogel | Rs (%) at Equilibrium State | |||||
|---|---|---|---|---|---|---|
| 20 °C | 40 °C | |||||
| pH = 4.0 | pH = 7.0 | pH = 9.0 | pH = 4.0 | pH = 7.0 | pH = 9.0 | |
| n-N-5/g-D60 | 3,720 | 3,220 | 940 | 2,370 | 670 | 150 |
| n-N-10/g-D60 | 2,580 | 2,000 | 480 | 1,910 | 400 | 120 |
| n-N-15/g-D60 | 1,890 | 1,520 | 290 | 1,140 | 110 | 100 |
| n-D-5/g-N60 | 4,750 | 3,030 | 720 | 3,240 | 1,500 | 170 |
| n-D-10/g-N60 | 4,090 | 1,770 | 460 | 3,030 | 660 | 130 |
| n-D-15/g-N60 | 3,360 | 1,320 | 290 | 2,680 | 180 | 120 |
3.6. Loading and in Vitro Release of Ceftriaxone Sodium

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, S.B.; Chu, L.Y.; Xu, D.; Zhang, J.; Ju, X.J.; Xie, R. Poly(N-isopropylacrylamide)-based comb-type grafted hydrogel with rapid response to blood glucose concentration change at physiological temperature. Polym. Adv. Tech. 2008, 19, 937–943. [Google Scholar] [CrossRef]
- Yashin, V.V.; Balazs, A.C. Pattern formation and shape changes in self-oscillating polymer gels. Science 2006, 314, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.; Kim, H.; Yoon, J. Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles. Sci. Rep. 2015, 5, 15124. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ober, C.K. Methods for the topographical patterning and patterned surface modification of hydrogels based on hydroxyethyl methacrylate. Biomacromolecules 2003, 4, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Cazacu, M.; Nistor, A. Ionic organic/inorganic materials. III. Stimuli responsive hybrid hydrogels based on oligo(N,N-dimethylaminoethylmethacrylate) and chloroalkyl-functionalized siloxanes. J. Polym. Sci. Part A 2009, 47, 6801–6813. [Google Scholar] [CrossRef]
- Kanekiyo, Y.; Sano, M.; Iguchi, R.; Shinkai, S. Novel nucleotide-responsive hydrogels designed from copolymers of boronic acid and cationic units and their applications as a QCM resonator system to nucleotide sensing. J. Polym. Sci. Part. A 2000, 38, 1302–1310. [Google Scholar] [CrossRef]
- Tang, Z.L.; Akiyama, Y.; Yamato, M.; Okano, T. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials 2010, 31, 7435–7443. [Google Scholar] [CrossRef] [PubMed]
- Champ, S.; Wei, X.; Huglin, M.B. Concentrating aqueous solutions of water soluble polymers by thermoreversible swelling of poly[(N-isopropylacrylamide)-co-(acrylic acid)] hydrogels. Macromol. Chem. Phys. 2000, 201, 931–940. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.S. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
- Akira, M.; Ryo, Y.; Kazunori, K. Glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety operating at the physiological pH. Biomacromolecules 2004, 5, 1038–1045. [Google Scholar]
- Okeyoshi, K.; Yoshida, R. Hydrogen generating gel systems induced by visible light. Soft Matter 2009, 5, 4118–4123. [Google Scholar] [CrossRef]
- Plunkett, K.N.; Berkowski, K.L.; Moore, J.S. Chymotrypsin responsive hydrogel: application of a disulfide exchange protocol for the preparation of methacrylamide containing peptides. Biomacromolecules 2005, 6, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, C.D.H.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, X.H.; Dong, C.M. Dual stimuli-responsive supramolecular polypeptide-based hydrogel and reverse micellar hydrogel mediated by host–guest chemistry. Adv. Funct. Mater. 2010, 20, 579–586. [Google Scholar] [CrossRef]
- Ebara, M.; Aoyagi, T.; Sakai, K.; Okano, T. Introducing reactive carboxyl side chains retains phase transition temperature sensitivity in N-isopropylacrylamide copolymer gels. Macromolecules 2000, 33, 8312–8316. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Kishi, R.; Hirasa, O.; Ichijo, H. Fast responsive poly(N-isopropylacrylamide) hydrogels prepared by γ-ray irradiation. Polym. Gels Netw. 1997, 5, 145–151. [Google Scholar] [CrossRef]
- Hasegawa, J.; Kanamori, K.; Nakanishi, K.; Hanada, T.; Yamago, S. Rigid crosslinked polyacrylamide monoliths with well-defined macropores synthesized by living polymerization. Macromol. Rapid Commun. 2009, 30, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.T.; Zheng, S.X. Poly(acrylic acid)-grafted Poly(N-isopropyl acrylamide) networks: Preparation, characterization and hydrogel behavior. J. Biomat. Sci. Polym. Ed. 2011, 22, 2305–2324. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xu, K.; Wang, P.; Li, W.; Sun, S.; Dong, L. High mechanical strength and rapid response rate of poly(N-isopropyl acrylamide) hydrogel crosslinked by starch-based nanospheres. Soft Matter 2010, 6, 1467–1471. [Google Scholar] [CrossRef]
- David, G.; Simionescu, B.C.; Albertsson, A.C. Rapid deswelling response of poly(N-isopropylacrylamide)/poly(2-alkyl-2-oxazoline)/poly(2-hydroxyethyl methacrylate) hydrogels. Biomacromolecules 2008, 9, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Yoshida, R.; Sakai, K.; Sakurai, Y.; Okano, T. Temperature-responsive shrinking kinetics of poly(N-isopropylacrylamide) copolymer gels with hydrophilic and hydrophobic comonomers. J. Membr. Sci. 1995, 101, 13–22. [Google Scholar] [CrossRef]
- Yoshida, R.; Uchida, K.; Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Comb-type grafted hydrogels with rapid de-swelling response to temperature changes. Nature 1995, 374, 240–242. [Google Scholar] [CrossRef]
- Kaneko, Y.; Sakai, K.; Kikuchi, A.; Yoshida, R.; Sakurai, Y.; Okano, T. Influence of freely mobile grafted chain length on dynamic properties of Comb-type grafted Poly(N-isopropylacrylamide) hydrogels. Macromolecules 1995, 28, 7717–7723. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T. Rapid deswelling response of poly(N-isopropylacrylamide) hydrogels by the formation of water release channels using poly(ethylene oxide) graft chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Annaka, M.; Tanaka, C.; Nakahira, T.; Sugiyama, M.; Aoyagi, T.; Okano, T. Fluorescence study on the swelling behavior of comb-type grafted poly(N-isopropylacrylamide) hydrogels. Macromolecules 2002, 35, 8173–9817. [Google Scholar] [CrossRef]
- González-Gómez, R.; Ortega, A.; Lazo, L.M.; Burillo, G. Retention of heavy metal ions on comb-type hydrogels based on acrylic acid and 4-vinylpyridine, synthesized by gamma radiation. Radiat. Phys. Chem. 2014, 102, 117–123. [Google Scholar]
- Burillo, G.; Castillo-Rojas, S.; Arrieta, H. Cu(II) immobilization in AAc/NIPAAm-based polymer systems synthesized using ionizing radiation. Radiat. Phys. Chem. 2012, 81, 278–283. [Google Scholar] [CrossRef]
- Illescas, J.; Burillo, G. pH- and temperature-responsive behavior of comb-type graft hydrogels of poly(acrylic acid) synthesized using gamma radiation. Macromol. Mat. Eng. 2009, 294, 414–421. [Google Scholar] [CrossRef]
- González, G.; Burillo, G. Synthesis of comb type and semi-interpenetrating networks of acryloyl-l-proline methyl ester and poly(acrylic acid) for Cu (II) immobilization. Radiat. Phys. Chem. 2010, 79, 870–875. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kowalewski, T.; Matyjaszewski, K. Comparison of thermoresponsive deswelling kinetics of poly(oligo(ethylene oxide) methacrylate)-based thermoresponsive hydrogels prepared by “graft-from” ATRP. Macromolecules 2011, 44, 2261–2268. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.B.; Kim, S.J.; Lee, Y.M. Rapid temperature/pH response of porous alginate-g-poly(N-isopropylacrylamide) hydrogels. Polymer 2002, 43, 7549–7558. [Google Scholar] [CrossRef]
- Ju, H.K.; Kim, S.Y.; Lee, Y.M. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacryamide). Polymer 2001, 42, 6851–6857. [Google Scholar] [CrossRef]
- Lee, S.B.; Ha, D.I.; Cho, S.K.; Kim, S.J.; Lee, Y.M. Temperature/pH-sensitive comb-type graft hydrogels composed of chitosan and poly(N-isopropylacrylamide). J. Appl. Polym. Sci. 2004, 92, 2612–2620. [Google Scholar] [CrossRef]
- Başer, B.; Demirel, G.B.; Açik, L.; Çaykara, T. Preparation of comb-type grafted hydrogels composed of polyacrylamide and chitosan and their use for DNA adsorption. J. Appl. Polym. Sci. 2009, 111, 1862–1868. [Google Scholar] [CrossRef]
- Annaka, M.; Matsuura, T.; Kasai, M.; Nakahira, T.; Hara, Y.; Okano, T. Preparation of comb-type N-isopropylacrylamide hydrogel beads and their application for size-selective separation media. Biomacromolecules 2003, 4, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Kikuchi, A.; Aoyagi, T.; Sakurai, Y.; Okano, T. Deswelling mechanism for comb-type grafted poly(N-isopropylacrylamide)hydrogelswith rapid temperature responses. Polym. Gels Networks 1998, 6, 333–345. [Google Scholar] [CrossRef]
- Chen, J.; Dai, P.P.; Liu, M.Z. Rapid responsive behaviors of the dual stimuli-sensitive poly(DEA-co-DMAEMA) hydrogel via comb-type grafted polymerization. Int. J. Polym. Mater. 2012, 61, 177–198. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, R.; Zhang, S.B.; Cheng, C.J.; Ju, X.J.; Chu, L.Y. Rapid pH/temperature-responsive cationic hydrogels with dual stimuli-sensitive grafted side chains. Polymer 2009, 50, 2516–2525. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Ma, L.; Chen, J. Thermo- and pH-sensitive comb-type grafted poly(N,N-diethylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Eur. Polym. J. 2009, 45, 2060–2067. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, L.-Y.; Li, Y.-K.; Lee, Y.M. Dual thermo- and pH-sensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Polymer 2007, 48, 1718–1728. [Google Scholar] [CrossRef]
- Pan, T.T.; He, W.D.; Li, L.Y.; Jiang, W.X.; He, C.; Tao, J. Dual thermo- and pH-sensitive network-grafted hydrogels formed by macrocrosslinker as drug delivery system. J. Polym. Sci. Part A 2011, 49, 2155–2164. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.Z.; Zhang, N.Y.; Dai, P.P.; Gao, C.M.; Liu, H.L. Influence of the grafted chain length on responsive behaviors of the grafted poly(DEA-co-DMAEMA) hydrogel. Sens. Actuators B 2010, 149, 34–43. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, P; Lu, M. Synthesis and swelling behavior of comb-type grafted hydrogels by reversible addition–fragmentation chain transfer polymerization. J. Polym. Sci. Part A 2005, 43, 2615–2624. [Google Scholar] [CrossRef]
- Ciampolini, M.; Nardi, N. Five-coordinated high-spin complexes of bivalent Cobalt, Nickel, and Copper with tris(2-dimethylaminoethyl)amine. Inorg. Chem. 1966, 5, 41–44. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Bossard, F.; Aubry, T.; Gotzamanis, G.; Tsitsilianis, C. pH-Tunable rheological properties of a telechelic cationic polyelectrolyte reversible hydrogel. Soft Matter 2006, 2, 510–516. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Q.; Li, J.-M.; Pan, T.-T.; Li, P.-Y.; He, W.-D. Comb-Type Grafted Hydrogels of PNIPAM and PDMAEMA with Reversed Network-Graft Architectures from Controlled Radical Polymerizations. Polymers 2016, 8, 38. https://doi.org/10.3390/polym8020038
Chen S-Q, Li J-M, Pan T-T, Li P-Y, He W-D. Comb-Type Grafted Hydrogels of PNIPAM and PDMAEMA with Reversed Network-Graft Architectures from Controlled Radical Polymerizations. Polymers. 2016; 8(2):38. https://doi.org/10.3390/polym8020038
Chicago/Turabian StyleChen, Sheng-Qi, Jia-Min Li, Ting-Ting Pan, Peng-Yun Li, and Wei-Dong He. 2016. "Comb-Type Grafted Hydrogels of PNIPAM and PDMAEMA with Reversed Network-Graft Architectures from Controlled Radical Polymerizations" Polymers 8, no. 2: 38. https://doi.org/10.3390/polym8020038
APA StyleChen, S. -Q., Li, J. -M., Pan, T. -T., Li, P. -Y., & He, W. -D. (2016). Comb-Type Grafted Hydrogels of PNIPAM and PDMAEMA with Reversed Network-Graft Architectures from Controlled Radical Polymerizations. Polymers, 8(2), 38. https://doi.org/10.3390/polym8020038