Influence of the Near Molecular Vicinity on the Temperature Regulated Fluorescence Response of Poly(N-vinylcaprolactam)
Abstract
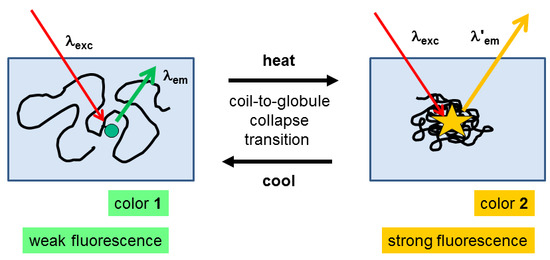
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. General
2.1.2. Synthesis of Intermediates
2-(2-hydroxyethyl)-6-morpholino-benzo[de]isoquinoline-1,3-dione (2)
6-morpholino-2-(2-(piperazin-1-yl)ethyl)-benzo[de]isoquinoline-1,3-dione (3)
4-(2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethoxy)-4-oxobut-2-enoic acid (4)
4-(4-(2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethyl)piperazin-1-yl)-4-oxobut-2-enoic acid (5)
2.1.3. Synthesis of Dye Monomers
4-(4-(2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethyl)piperazin-1-yl)-N-octadecyl-4-oxobut-2-enamide (1a)
N-((-adamantan-1-yl)methyl)-4-(4-(2-(6-morpholino-1,3-dioxo-benzo[de]iso-quinolin-2-yl)ethyl)piperazin-1-yl)-4-oxobut-2-enamide (1b)
N-butyl-4-(4-(2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethyl)piperazin-1-yl)-4-oxobut-2-enamide (1c)
6-morpholino-2-(2-(4-(4-oxo-4-(piperidin-1-yl)but-2-enoyl)piperazin-1-yl)ethyl)-benzo[de]isoquinoline-1,3-dione (1d)
2-(acetoxymethyl)-6-(2-(4-((-4-(4-(2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethyl)piperazin-1-yl)-4-oxobut-2-enamido)methyl)-1,2,3-triazol-1-yl)ethoxy)tetrahydro-pyran-3,4,5-triyl triacetate (1e)
6-(((-4-(4-(2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethyl)piperazin-1-yl)-4-oxobut-2-enoyl)oxy)methyl)tetrahydro-pyran-2,3,4,5-tetrayl tetraacetate (1f)
2-(6-morpholino-1,3-dioxo-benzo[de]isoquinolin-2-yl)ethyl-4-(((-adamantan-1-yl)-methyl)-amino)- 4-oxobut-2-enoate (1g)
2.1.4. Synthesis of the Polymers
2.2. Methods
3. Results and Discussion
3.1. Synthesis of the Fluorophore-Labeled Polymers
3.2. Spectroscopic Properties of the Monomers and Polymers In Ethanol
3.3. Thermoresponsive Behavior of the Dye-Labeled Polymers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Wischerhoff, E.; Badi, N.; Lutz, J.-F.; Laschewsky, A. Smart Bioactive Surfaces. Soft Matter 2010, 6, 705–713. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F. Non-ionic thermoresponsive polymers in water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Bioinspired poly(2-oxazoline)s. Polymers 2011, 3, 467–488. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Aseyev, V.; Tenhu, H.; Winnik, F. Temperature dependence of the colloidal stability of neutral amphiphilic polymers in water. Adv. Polym. Sci. 2006, 196, 1–85. [Google Scholar]
- Ringsdorf, H.; Venzmer, J.; Winnik, F. Interaction of hydrophobically-modified poly-N-isopropylacrylamides with model membranes—Or playing a molecular accordion. Angew. Chem. Int. Ed. 1991, 30, 315–318. [Google Scholar] [CrossRef]
- Mertoglu, M.; Garnier, S.; Laschewsky, A.; Skrabania, K.; Storsberg, J. Stimuli responsive amphiphilic block copolymers for aqueous media synthesised via reversible addition fragmentation chain transfer polymerisation (RAFT). Polymer 2005, 46, 7726–7740. [Google Scholar] [CrossRef]
- Birnbaum, W.; Kuckling, D. Synthesis of α-biotinyl poly(ethylene glycol-b-N-isopropylacrylamide) block copolymers with different fluorescent dyes at the ω-side. Polym. Chem. 2012, 3, 2039–2049. [Google Scholar] [CrossRef]
- Hiruta, Y.; Shimamura, M.; Matsuura, M.; Maekawa, Y.; Funatsu, T.; Suzuki, Y.; Ayano, E.; Okano, T.; Kanazawa, H. Temperature-responsive fluorescence polymer probes with accurate thermally controlled cellular uptakes. ACS Macro Lett. 2014, 3, 281–285. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ottaviani, M.F.; Bossman, S.H.; Pan, W.; Garcia-Garibay, M.; Turro, N.J. Phase separation of poly(N-isopropylacrylamide) in water: A spectroscopic study of a polymer tagged with a fluorescent dye and a spin label. J. Phys. Chem. 1993, 97, 12998–13005. [Google Scholar] [CrossRef]
- Laukkanen, A.; Winnik, F.M.; Tenhu, H. Pyrene-labeled graft copolymers of N-vinylcaprolactam: Synthesis and solution properties in water. Macromolecules 2005, 38, 2439–2448. [Google Scholar] [CrossRef]
- Yoshinari, E.; Furukawa, H.; Horie, K. Fluorescence study on the mechanism of rapid shrinking of grafted poly(N-isopropylacrylamide) gels and semi-IPN gels. Polymer 2005, 46, 7741–7748. [Google Scholar] [CrossRef]
- Chee, C.K.; Rimmer, S.; Soutar, I.; Swanson, L. Fluorescence investigations of the conformational behaviour of poly(N-vinylcaprolactam). React. Funct. Polym. 2006, 66, 1–11. [Google Scholar] [CrossRef]
- Matsumura, Y.; Katoh, A. Synthesis of 2,3-dimorpholino-6-aminoquinoxaline derivatives and application to a new intramolecular fluorescent probe. J. Lumin. 2008, 128, 625–630. [Google Scholar] [CrossRef]
- Yusa, S.-I.; Endo, T.; Ito, M. Synthesis of thermo-responsive 4-arm star-shaped porphyrin-centered poly(N,N-diethylacrylamide) via reversible addition-fragmentation chain transfer radical polymerization. J. Polym. Sci. A Polym. Chem. 2009, 47, 6827–6838. [Google Scholar] [CrossRef]
- Nagai, A.; Kokado, K.; Miyake, J.; Cyujo, Y. Thermoresponsive fluorescent water-soluble copolymers containing BODIPY dye: Inhibition of H-aggregation of the BODIPY units in their copolymers by LCST. J. Polym. Sci. A Polym. Chem. 2010, 48, 627–634. [Google Scholar] [CrossRef]
- Weiss, J.; Laschewsky, A. Temperature induced self-assembly of triple responsive triblock copolymers in aqueous solutions. Langmuir 2011, 27, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Thivaios, I.; Diamantis, I.; Bokias, G.; Kallitsis, J.K. Temperature-responsive photoluminescence of quinoline-labeled poly(N-isopropylacrylamide) in aqueous solution. Eur. Polym. J. 2012, 48, 1256–1265. [Google Scholar] [CrossRef]
- Inal, S.; Kölsch, J.D.; Chiappisi, L.; Janietz, D.; Gradzielski, M.; Laschewsky, A.; Neher, D. Structure-related differences in the temperature regulated fluorescence response of LCST type polymers. J. Mater. Chem. C 2013, 1, 6603–6612. [Google Scholar] [CrossRef]
- Jordão, N.; Gavara, R.; Parola, A.J. Flavylium-supported poly(N-isopropylacrylamide): A class of multistimuli responsive polymer. Macromolecules 2013, 46, 9055–9063. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, H.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Thermo- and light-regulated formation and disintegration of double hydrophilic block copolymer assemblies with tunable fluorescence emissions. Langmuir 2013, 29, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Zhang, B.; Kuo, C.-Y.; Martinez, J.S.; Park, J.; Mallapragada, S.; Wang, H.-L. Stimuli-responsive poly-N-isopropylacrylamide: Phenylene vinylene Oligomer Conjugate. J. Phys. Chem. C 2013, 117, 7757–7763. [Google Scholar] [CrossRef]
- Uchiyama, S.; Matsumura, Y.; de Silva, A.P.; Iwai, K. Fluorescent molecular thermometers based on polymers showing temperature-induced phase transitions and labeled with polarity-responsive benzofurazans. Anal. Chem. 2003, 75, 5926–5935. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Matsumura, Y.; Prasanna de Silva, A.; Iwai, K. Modulation of the sensitive temperature range of fluorescent molecular thermometers based on thermoresponsive polymers. Anal. Chem. 2004, 76, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Matsumura, Y.; Uchiyama, S.; Prasanna de Silva, A. Development of fluorescent microgel thermometers based on thermo-responsive polymers and their modulation of sensitivity range. J. Mater. Chem. 2005, 15, 2796–2800. [Google Scholar] [CrossRef]
- Matsumura, Y.; Iwai, K. Synthesis and thermo-responsive behavior of fluorescent labeled microgel particles based on poly(N-isopropylacrylamide) and its related polymers. Polymer 2005, 46, 10027–10034. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Miyamoto, R.; Zhang, X.; Hirai, T. Rhodamine-based fluorescent thermometer exhibiting selective emission enhancement at a specific temperature range. Org. Lett. 2007, 9, 3921–3924. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Miyamoto, R.; Hirai, T. A Hemicyanine-conjugated copolymer as a highly sensitive fluorescent thermometer. Langmuir 2008, 24, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Gota, C.; Uchiyama, S.; Yoshihara, T.; Tobita, S.; Ohwada, T. Temperature-dependent fluorescence lifetime of a fluorescent polymeric thermometer, poly(N-isopropylacrylamide), Labeled by polarity and hydrogen bonding sensitive 4-sulfamoyl-7-aminobenzofurazan. J. Phys. Chem. B 2008, 112, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, W.; Xiong, Y.; Tian, H. Multiple logic fluorescent thermometer system based on N-isopropylmethacrylamide copolymer bearing dicyanomethylene-4H-pyran moiety. Macromolecules 2009, 42, 1448–1453. [Google Scholar] [CrossRef]
- Pietsch, C.; Hoogenboom, R.; Schubert, U.S. Soluble polymeric dual sensor for temperature and pH value. Angew. Chem. Int. Ed. 2009, 48, 5653–5656. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Miyamoto, R.; Shiraishi, Y.; Hirai, T. BODIPY-conjugated thermoresponsive copolymer as a fluorescent thermometer based on polymer microviscosity. Langmuir 2009, 25, 13176–13182. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Yoshii, R.; Otsuka, T.; Kokado, K.; Chujo, Y. BODIPY-based chain transfer agent: Reversibly thermoswitchable luminescent gold nanoparticle stabilized by BODIPY-terminated water-soluble polymer. Langmuir 2010, 26, 15644–15649. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, C.-T. A PNIPAM-based fluorescent nanothermometer with ratiometric readout. Chem. Commun. 2011, 47, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, X.; Wang, D.; Hu, X.; Liu, T.; Zhang, G.; Liu, S. Ultrasensitive ratiometric fluorescent pH and temperature probes constructed from dye-labeled thermoresponsive double hydrophilic block copolymers. J. Mater. Chem. 2011, 21, 19030–19038. [Google Scholar] [CrossRef]
- Uchiyama, S.; Kimura, K.; Gota, C.; Okabe, K.; Kawamoto, K.; Inada, N.; Yoshihara, T.; Tobita, S. Environment-sensitive fluorophores with benzothiadiazole and benzoselenadiazole structures as candidate components of a fluorescent polymeric thermometer. Chem. Eur. J. 2012, 18, 9552–9563. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, X.; Xue, W.; Huang, S.; Dong, J.; Wei, L.; Maroncelli, M.; Li, H. Synthesis, structures, and properties of a fluoranthene-based biphenol polymer as a fluorescent nano-thermometer. Chem. Eng. J. 2014, 240, 319–330. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, C.; Qi, L.; Liu, M.; Dong, P.; Jiang, Q.; Yang, X.; Mu, X.; Mao, L. Intracellular temperature sensing by a ratiometric fluorescent polymer thermometer. J. Mater. Chem. B 2014, 2, 7544–7550. [Google Scholar] [CrossRef]
- Zhou, J.; Mishra, K.; Bhagat, V.; Joy, A.; Becker, M.L. Thermoresponsive dual emission nanosensor based on quantum dots and dye labeled poly(N-isopropylacrylamide). Polym. Chem. 2015, 6, 2813–2816. [Google Scholar] [CrossRef]
- Malfait, A.; Coumes, F.; Fournier, D.; Cooke, G.; Woisel, P. A water-soluble supramolecular polymeric dual sensor for temperature and pH with an associated direct visible readout. Eur. Polym. J. 2015, 69, 552–558. [Google Scholar] [CrossRef]
- Vancoillie, G.; Zhang, Q.; Hoogenboom, R. Chapter 7: Polymeric temperature sensors. In Thermometry at the Nanoscale: Techniques and Selected Applications; The Royal Society of Chemistry: Cambride, UK, 2016; pp. 190–236. [Google Scholar]
- Koopmans, C.; Ritter, H. Color change of N-isopropylacrylamide copolymer bearing reichardts dye as optical sensor for lower critical solution temperature and for host-guest interaction with β-cyclodextrin. J. Am. Chem. Soc. 2007, 129, 3502–3503. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dai, L.; Liu, S. Analyte-reactive amphiphilic thermoresponsive diblock copolymer micelles-based multifunctional ratiometric fluorescent chemosensors. Macromolecules 2011, 44, 4699–4710. [Google Scholar] [CrossRef]
- Pietsch, C.; Schubert, U.S.; Hoogenboom, R. Aqueous polymeric sensors based on temperature-induced polymer phase transitions and solvatochromic dyes. Chem. Commun. 2011, 47, 8750–8765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, F.; Tian, Y.; Johnson, R.H.; Meldrum, D.R. Platinum (II) porphyrin-containing thermoresponsive poly(N-isopropylacrylamide) copolymer as fluorescence dual oxygen and temperature sensor. Sens. Actuators. B Chem. 2011, 159, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Inal, S.; Chiappisi, L.; Kölsch, J.D.; Kraft, M.; Appavou, M.-S.; Scherf, U.; Wagner, M.; Hansen, M.R.; Gradzielski, M.; Laschewsky, A.; et al. Temperature-regulated fluorescence and association of an oligo(ethyleneglycol)-methacrylate-based copolymer with a conjugated polyelectrolyte—The effect of solution ionic strength. J. Phys. Chem. B 2013, 117, 14576–14587. [Google Scholar] [CrossRef] [PubMed]
- Inal, S.; Kölsch, J.D.; Sellrie, F.; Schenk, J.A.; Wischerhoff, E.; Laschewsky, A.; Neher, D. A water soluble fluorescent polymer as a dual colour sensor for temperature and a specific protein. J. Mater. Chem. B 2013, 1, 6373–6381. [Google Scholar] [CrossRef]
- Seeboth, A.; Lötzsch, D.; Ruhmann, R.; Muehling, O. Thermochromic polymers—Function by design. Chem. Rev. 2014, 114, 3037–3068. [Google Scholar] [CrossRef] [PubMed]
- Eisele, M.; Burchard, W. Hydrophobic water-soluble polymers, 1. Dilute solution properties of poly(1-vinyl-2-piperidone) and poly(N-vinylcaprolactam). Makromol. Chem. 1990, 191, 169–184. [Google Scholar] [CrossRef]
- Tager, A.A.; Safronov, A.P.; Sharina, S.V.; Galaev, I.Y. Thermodynamic study of poly(N-vinyl caprolactam) hydration at temperatures close to lower critical solution temperature. Colloid Polym. Sci. 1993, 271, 868–872. [Google Scholar] [CrossRef]
- Meeussen, F.; Nies, E.; Berghmans, H.; Verbrugghe, S.; Goethals, E.; Du Prez, F. Phase behaviour of poly(N-vinyl caprolactam) in water. Polymer 2000, 41, 8597–8602. [Google Scholar] [CrossRef]
- Aseyev, V.; Hietala, S.; Laukkanen, A.; Nuopponen, M.; Confortini, O.; Du Prez, F.E.; Tenhu, H. Mesoglobules of thermoresponsive polymers in dilute aqueous solutions above the LCST. Polymer 2005, 46, 7118–7131. [Google Scholar] [CrossRef]
- Zhao, X.; Coutelier, O.; Nguyen, H.H.; Delmas, C.; Destarac, M.; Marty, J.-D. Effect of copolymer composition of RAFT/MADIX-derived N-vinylcaprolactam/N-vinylpyrrolidone statistical copolymers on their thermoresponsive behavior and hydrogel properties. Polym. Chem. 2015, 6, 5233–5243. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Simenel, I.A.; Kurskaya, E.A.; Kulakova, V.K.; Galaev, I.Y.; Mattiasson, B.; Grinberg, V.Y.; Grinberg, N.V.; Khokhlov, A.R. Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer 2000, 41, 3737–3743. [Google Scholar] [CrossRef]
- Ieong, N.S.; Hasan, M.; Phillips, D.J.; Saaka, Y.; O’Reilly, R.K.; Gibson, M.I. Polymers with molecular weight dependent LCSTs are essential for cooperative behaviour. Polym. Chem. 2012, 3, 794–799. [Google Scholar] [CrossRef]
- Shao, L.; Hu, M.; Chen, L.; Xu, L.; Bi, Y. RAFT polymerization of N-vinylcaprolactam and effects of the end group on the thermal response of poly(N-vinylcaprolactam). React. Funct. Polym. 2012, 72, 407–413. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Anton, P.; Laschewsky, A. Zwitterionic polysoaps with reduced density of surfactant side groups. Makromol. Chem. 1993, 194, 601–624. [Google Scholar] [CrossRef]
- El-Guweri, M.; Hendlinger, P.; Laschewsky, A. Partially fluorinated maleimide copolymers for Langmuir films of improved stability, 1. Synthesis, copolymerisation behaviour and bulk properties. Macromol. Chem. Phys. 1997, 198, 401–418. [Google Scholar] [CrossRef]
- Auzély-Velty, R.; Cristea, M.; Rinaudo, M. Galactosylated N-vinylpyrrolidone-maleic acid copolymers: Synthesis, characterization, and interaction with lectins. Biomacromolecules 2002, 3, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Prisacaru, A.I.; Suflet, D.M.; Fundueanu, G. Thermo- and pH-sensitivity of poly(N-vinylcaprolactam-co-maleic acid) in aqueous solution. Polym. Bull. 2014, 71, 2863–2880. [Google Scholar] [CrossRef]
- Dahmén, J.; Frejd, T.; Grönberg, G.; Lave, T.; Magnusson, G.; Noori, G. 2-Bromoethyl glycosides: Synthesis and characterisation. Carbohydr. Res. 1983, 116, 303–307. [Google Scholar] [CrossRef]
- Kleinert, M.; Röckendorf, N.; Lindhorst, T.K. Glyco-SAMs as glycocalyx mimetics: Synthesis of L-Fucose- and D-Mannose-terminated building blocks. Eur. J. Org. Chem. 2004, 2004, 3931–3940. [Google Scholar] [CrossRef]
- Wu, J.; Yi, T.; Shu, T.; Yu, M.; Zhou, Z.; Xu, M.; Zhou, Y.; Zhang, H.; Han, J.; Li, F.; et al. Ultrasound switch and thermal self-repair of morphology and surface wettability in a cholesterol-based self-assembly system. Angew. Chem., Int. Ed. 2008, 47, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, J.; He, Y.; Yang, J.H.; Kim, T.; Peng, X.; Kim, J.S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4601. [Google Scholar] [CrossRef] [PubMed]











| Polymer | Comonomer | Yield (%) | Dye monomer incorporated (mol %) a | Mn app (kDa) b | Mw app (kDa) b | Average No. of fluorophores per polymer chain |
|---|---|---|---|---|---|---|
| PNVCL-0 | - | 82 c | - | 30 | 72 | - |
| PNVCL-a | 1a | 72 d | 0.10 | 30 | 119 | 0.2 |
| PNVCL-b | 1b | 82 d | 0.09 | 24 | 100 | 0.2 |
| PNVCL-c | 1c | 76 d | 0.12 | 47 | 125 | 0.4 |
| PNVCL-d | 1d | 70 d | 0.10 | 21 | 75 | 0.2 |
| PNVCL-e | 1e | 70 d | 0.11 | 48 | 117 | 0.4 |
| PNVCL-f | 1f | 80 c | 0.07 | 26 | 93 | 0.1 |
| PNVCL-g | 1g | 80 c | 0.16 | 25 | 86 | 0.3 |
| Dye monomer | λmaxAbs (EtOH) (nm) | ε (EtOH) (L·mol−1·cm−1) | λmaxEm (EtOH) (nm) | Φ (EtOH) (%) |
|---|---|---|---|---|
| 1a | 396 | 10,000 | 532 | 0.09 |
| 1b | 398 | 11,000 | 532 | 0.07 |
| 1c | 396 | 8,800 | 530 | 0.07 |
| 1d | 396 | 9,700 | 531 | 0.08 |
| 1e | 395 | 9,700 | 532 | 0.09 |
| 1f | 395 | 9,400 | 531 | 0.08 |
| 1g | 398 | 9,400 | 534 | 0.06 |
| Polymer | λmaxAbs (EtOH) (nm) | λmaxEm (EtOH) (nm) | λmaxAbs (H2O) (nm) | λmaxEm (H2O) (nm) |
|---|---|---|---|---|
| PNVCL-a | 397 | 530 | 407 | 534 |
| PNVCL-b | 397 | 530 | 410 | 539 |
| PNVCL-c | 398 | 530 | 410 | 541 |
| PNVCL-d | 397 | 530 | 410 | 541 |
| PNVCL-e | 397 | 530 | 407 | 538 |
| PNVCL-f | 395 | 530 | 409 | 541 |
| PNVCL-g | 400 | 529 | 410 | 541 |
| Polymer | λmaxAbs (nm) | λmaxEm at 10 °C (nm) | λmaxEm at 60 °C (nm) | Δλmax10–60 °C (nm) a | IT=60°C/IT=10°C b |
|---|---|---|---|---|---|
| PNVCL-a | 407 | 536 | 525 | 11 | 3.3 |
| PNVCL-b | 410 | 541 | 525 | 16 | 5.7 |
| PNVCL-c | 410 | 542 | 526 | 16 | 6.6 |
| PNVCL-d | 410 | 543 | 527 | 16 | 7.2 |
| PNVCL-e | 407 | 538 | 526 | 12 | 8.0 |
| PNVCL-f | 408 | 543 | 525 | 18 | 6.7 |
| PNVCL-g | 410 | 541 | 532 | 10 | 1.2 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enzenberg, A.; Laschewsky, A.; Boeffel, C.; Wischerhoff, E. Influence of the Near Molecular Vicinity on the Temperature Regulated Fluorescence Response of Poly(N-vinylcaprolactam). Polymers 2016, 8, 109. https://doi.org/10.3390/polym8040109
Enzenberg A, Laschewsky A, Boeffel C, Wischerhoff E. Influence of the Near Molecular Vicinity on the Temperature Regulated Fluorescence Response of Poly(N-vinylcaprolactam). Polymers. 2016; 8(4):109. https://doi.org/10.3390/polym8040109
Chicago/Turabian StyleEnzenberg, Anne, André Laschewsky, Christine Boeffel, and Erik Wischerhoff. 2016. "Influence of the Near Molecular Vicinity on the Temperature Regulated Fluorescence Response of Poly(N-vinylcaprolactam)" Polymers 8, no. 4: 109. https://doi.org/10.3390/polym8040109
APA StyleEnzenberg, A., Laschewsky, A., Boeffel, C., & Wischerhoff, E. (2016). Influence of the Near Molecular Vicinity on the Temperature Regulated Fluorescence Response of Poly(N-vinylcaprolactam). Polymers, 8(4), 109. https://doi.org/10.3390/polym8040109