PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mateirals
2.2. Synthesis of Carboxylic Acid-Terminated Methoxypolyethylene Glycol (mPEG-Acid)
2.3. Synthesis of Methoxypolyethylene Glycol-g-Chitosan (mPEG-g-Chitosan)
2.4. 1H Nuclear Magnetic Resonance Spectroscopy (1H NMR) Analysis
2.5. Rheological Analysis
2.6. Scanning Electron Microscopy (SEM) Evaluation of Polymer Morphology
2.7. Cell Proliferation Analysis
2.8. Dose-Dependent Cytotoxicity Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Structure of mPEG-g-Chitosan
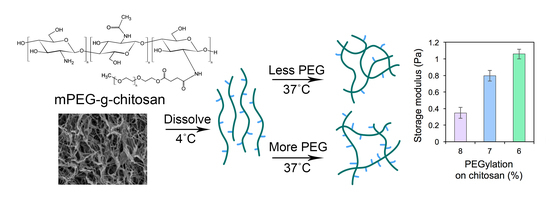
3.2. Rheological Properties and Stiffness of mPEG-g-Chitosan Hydrogels
3.2.1. Thermally-Induced Gelation
3.2.2. Hydrogel Mechanical Properties
3.2.3. Hydrogel Restoration
3.3. Morphology of Lyophilized mPEG-g-Chitosan
3.4. MMC Cell Behavior on mPEG-g-Chitosan Hydrogels of Varying Stiffness
3.4.1. MMC Cell Proliferation and Morphology
3.4.2. MMC Cell Response to Doxorubicin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tse, J.R.; Engler, A.J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 2010. [Google Scholar] [CrossRef]
- DeForest, C.A.; Anseth, K.S. Advances in bioactive hydrogels to probe and direct cell fate. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, S.; Khademhosseini, A.; Yang, Y. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials 2011, 32, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Jana, S.; Tsao, C.T.; Zhang, M. Tenogenic differentiation of human bone marrow stem cells via a combinatory effect of aligned chitosan–poly-caprolactone nanofibers and TGF-β3. J. Mater. Chem. B 2013, 1, 6516–6524. [Google Scholar] [CrossRef]
- Leung, M.; Cooper, A.; Jana, S.; Tsao, C.T.; Petrie, T.A.; Zhang, M. Nanofiber-based in vitro system for high myogenic differentiation of human embryonic stem cells. Biomacromolecules 2013, 14, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling self-renewal and differentiation of stem cells via mechanical cues. J. Biomed. Biotechnol. 2012, 2012, 797410–797412. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.V. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhu, C.; Guo, Q.; Yang, H.; Li, B. Cellular modulation by the elasticity of biomaterials. J. Mater. Chem. B. 2016, 4, 9–26. [Google Scholar] [CrossRef]
- Watt, F.M.; Huck, W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Seewaldt, V. ECM stiffness paves the way for tumor cells. Nat. Med. 2014, 20, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Yui, Y.; Damiano, L.; Bainer, R.O.; Lakins, J.N.; Acerbi, I.; Ou, G.; Wijekoon, A.C.; Levental, K.R.; Gilbert, P.M.; et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 2014, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, T.A.; Jain, A.; Tanner, K.; MacKay, J.L.; Kumar, S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 2010, 31, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Peyton, S.R.; Raub, C.B.; Keschrumrus, V.P.; Putnam, A.J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 2006, 27, 4881–4893. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, R.W.; Cowan, C.R.; Mih, J.D.; Koryakina, Y.; Gioeli, D.; Slack-Davis, J.K.; Blackman, B.R.; Tschumperlin, D.J.; Parsons, J.T. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Nihouannen, L.D.; Hacking, S.A.; Tran, S.; Li, J.; Murshed, M.; Doillon, C.J.; Ghezzi, C.E.; Zhang, Y.L.; Nazhat, S.N.; et al. Newly identified interfibrillar collagen crosslinking suppresses cell proliferation and remodelling. Biomaterials 2015, 54, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Tsao, C.T.; Chang, C.H.; Lai, Y.T.; Wu, M.F.; Chuang, C.N.; Chou, H.C.; Wang, C.K.; Hsieh, K.H. Assessment of reinforced poly(ethylene glycol) chitosan hydrogels as dressings in a mouse skin wound defect model. Mater. Sci. Eng. C 2013, 33, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Tsao, C.T.; Chang, C.H.; Lai, Y.-T.; Wu, M.F.; Liu, Z.W.; Chuang, C.N.; Chou, H.C.; Wang, C.K.; Hsieh, K.H. Synthesis and characterization of reinforced poly(ethylene glycol)/chitosan hydrogel as wound dressing materials, macromol. Mater. Eng. 2013, 298, 429–438. [Google Scholar]
- Tsao, C.T.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. Chitosan-based thermoreversible hydrogel as an in vitro tumor microenvironment for testing breast cancer therapies. Mol. Pharm. 2014, 11, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Kievit, F.M.; Ravanpay, A.; Erickson, A.E.; Jensen, M.C.; Ellenbogen, R.G.; Zhang, M. Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic t lymphocyte depot for localized glioblastoma immunotherapy. Biomacromolecules 2014, 15, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Leung, M.; Chang, J.Y.F.; Zhang, M. A simple material model to generate epidermal and dermal layers in vitro for skin regeneration. J. Mater. Chem. B 2014, 2, 5256–5264. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Hsiao, M.H.; Zhang, M.Y.; Levengood, S.L.; Zhang, M. Chitosan-PEG hydrogel with sol-gel transition triggerable by multiple external stimuli, macromol. Rapid Commun. 2014, 36, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Li, Y.D.; Wu, M.F.; Lin, C.P.; Han, J.L.; Chen, S.H.; Hsieh, K.H. Development of chitosan/dicarboxylic acid hydrogels as wound dressing materials. J. Bioact. Compat. Polym. 2011, 26, 519–536. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsao, C.T.; Chang, K.Y.; Wang, J.L.; Young, T.H.; Han, J.L.; Hsieh, K.H. Chitosan membrane with surface-bonded growth factor in guided tissue regeneration applications. J. Bioact. Compat. Polym. 2010, 25, 465–482. [Google Scholar] [CrossRef]
- Chen, S.H.; Tsao, C.T.; Chang, C.H.; Wu, Y-M.; Liu, Z.W.; Lin, C.P.; Wang, C.K.; Hsieh, K.H. Synthesis and characterization of thermal-responsive chitin-based polyurethane copolymer as a smart material. Carbohydr. Polym. 2012, 88, 1483–1487. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.Y.; Zhu, Y.J.; Liu, P.F.; Zhu, M.J.; Duan, Y.R. Synthesis of calcium phosphate/GPC-mPEG hybrid porous nanospheres for drug delivery to overcome multidrug resistance in human breast cancer. J. Mater. Chem. 2012, 22, 5128–5136. [Google Scholar] [CrossRef]
- Abraham, R.J.; Byrne, J.J.; Griffiths, L.; Perez, M. 1H chemical shifts in NMR: Part 23, the effect of dimethyl sulphoxide versus chloroform solvent on 1H chemical shifts. Magn. Reson. Chem. 2006, 44, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Torres, D.; Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Alonso, M.J. Chitosan-PEG nanocapsules as new carriers for oral peptide delivery. Effect of chitosan pegylation degree. J. Controll. Release 2006, 111, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, X.; Shi, S.; Gu, Y.; Yang, L.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. Biodegradable MPEG-g-chitosan and methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) composite films: Part 1. Prep. Charact. Carbohydr. Polym. 2010, 79, 429–436. [Google Scholar] [CrossRef]
- Tsao, C.T.; Hsiao, M.H.; Zhang, M.Y.; Levengood, S.L.; Zhang, M. Chitosan-PEG Hydrogel with sol–gel transition triggerable by multiple external stimuli. Macromol. Rapid Commun. 2015, 36, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar]
- Carstens, M.G.; van Nostrum, C.F.; Ramzi, A.; Meeldijk, J.D.; Verrijk, R.; de Leede, L.L.; Crommelin, D.J.A.; Hennink, W.E. Poly(ethylene glycol)−oligolactates with monodisperse hydrophobic blocks: Preparation, characterization, and behavior in water. Am. Chem. Soc. 2005. [Google Scholar] [CrossRef]
- Ganji, F.; Abdekhodaie, M.J. Synthesis and characterization of a new thermosensitive chitosan–PEG diblock copolymer. Carbohydr. Polym. 2008, 74, 435–441. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuda, E.Y.; Leight, J.L.; Anseth, K.S. Modulation of matrix elasticity with PEG hydrogels to study melanoma drug responsiveness. Biomaterials 2014, 35, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.I.; Abaci, H.E.; Krupski, Y.; Weng, L.C.; Burdick, J.A.; Gerecht, S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater. Sci. 2014, 2, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; Hammer, D.A.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Zhao, S.; Wang, J.; Liu, G.; Du, Y. Micro-scaffold array chip for upgrading cell-based high-throughput drug testing to 3D using benchtop equipment. Lab Chip. 2014, 14, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jeong, J.; DeVolder, R.J.; Cha, C.; Wang, F.; Tong, Y.W.; Kong, H. A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials 2011, 32, 9308–9315. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.L.; Bissell, M.J. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist. Updat. 2012, 15, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.J.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Rubashkin, M.G.; Cassereau, L.; Bainer, R.; DuFort, C.C.; Yui, Y.; Ou, G.; Paszek, M.J.; Davidson, M.W.; Chen, Y.Y.; Weaver, V.M. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res. 2014, 74, 4597–4611. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed]







| Sample | mPEG-acid (g) | Chitosan (g) | EDC (g) | NHS (g) |
|---|---|---|---|---|
| mPEG-g5-Chitosan | 0.43 | 0.50 | 0.20 | 0.12 |
| mPEG-g6-Chitosan | 0.43 | 0.45 | 0.20 | 0.12 |
| mPEG-g7-Chitosan | 0.43 | 0.40 | 0.20 | 0.12 |
| mPEG-g8-Chitosan | 0.43 | 0.35 | 0.20 | 0.12 |
| mPEG-g11-Chitosan | 0.43 | 0.30 | 0.20 | 0.12 |
| mPEG-g13-Chitosan | 0.43 | 0.25 | 0.20 | 0.12 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, F.-C.; Tsao, C.-T.; Lin, A.; Zhang, M.; Levengood, S.L.; Zhang, M. PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells. Polymers 2016, 8, 112. https://doi.org/10.3390/polym8040112
Chang F-C, Tsao C-T, Lin A, Zhang M, Levengood SL, Zhang M. PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells. Polymers. 2016; 8(4):112. https://doi.org/10.3390/polym8040112
Chicago/Turabian StyleChang, Fei-Chien, Ching-Ting Tsao, Anqi Lin, Mengying Zhang, Sheeny Lan Levengood, and Miqin Zhang. 2016. "PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells" Polymers 8, no. 4: 112. https://doi.org/10.3390/polym8040112
APA StyleChang, F. -C., Tsao, C. -T., Lin, A., Zhang, M., Levengood, S. L., & Zhang, M. (2016). PEG-Chitosan Hydrogel with Tunable Stiffness for Study of Drug Response of Breast Cancer Cells. Polymers, 8(4), 112. https://doi.org/10.3390/polym8040112