Studies of Grafted and Sulfonated Spiro Poly(isatin-ethersulfone) Membranes by Super Acid-Catalyzed Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of 4,4-(2,6-dimethylphenyloxy)Diphenyl Sulfone (DMPPS)
2.3. Preparation of Poly(isatin-ethersulfone)
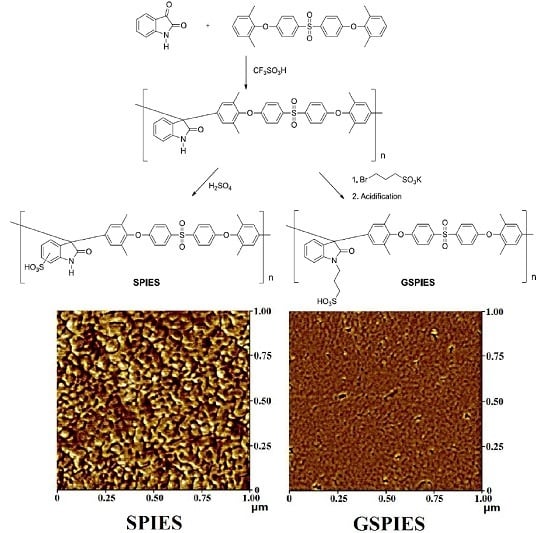
2.4. Synthesis of Sulfonated Poly(isatin-ethersulfone) (SPIES)
2.5. Synthesis of 3-bromo-1-propanesulfonic Acid Potassium Salt
2.6. Synthesis of Grafting Sulfonated Poly(isatin-ethersulfone) (GSPIES)
2.7. Membrane Preparation and Characterization
3. Results and Discussion
3.1. Charaterization of Monomer and Polymers
3.2. Properties of Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Savadogo, O. Emerging membrames for electrochemical systems: (I) Solid polymer electrolyte membranes for fuel cell systems. J. New Mater. Electr. Syst. 1998, 1, 47–66. [Google Scholar]
- Roziere, J.; Jones, D.J. Non-fluorinated polymer materials for proton exchange membrane fuel cells. Rev. Mater. Res. 2003, 33, 503–555. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Luo, J.; Jensen, A.H.; Brooks, N.R.; Sniekers, J.; Knipper, M.; Aili, D.; Li, Q.; Vanroy, B.; Wübbenhorst, M.; Yan, F.; et al. 1,2,4-Triazolium perfluorobutanesulfonate as an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells. Energy Env. Sci. 2015, 8, 1276–1291. [Google Scholar] [CrossRef]
- Luo, J.; Conrad, O.; Vankelecom, I.J. Imidazolium methansulfonate as a high temperature proton conductor. J. Mater. Chem. A 2013, 1, 2238–2247. [Google Scholar] [CrossRef]
- Luo, J.; Hu, J.; Saak, W.; Beckhaus, R.; Wittstock, G.; Vankelecom, I.J.; Aqert, C.; Conrad, O. Protic ionic liquid and ionic melts prepared from methansulfonic acid and 1H-1,2,4-triazole as high temperature PEMFC electrolytes. J. Mater. Chem. 2011, 21, 10426–10436. [Google Scholar] [CrossRef]
- Allcock, H.R.; Hofmann, M.A.; Ambler, C.M.; Lvov, S.N.; Zhou, X.Y.; Chalkova, E. Phenyl phosphonic acid functionalized poly[aryloxyphosphazenes] as proton-conducting membranes for direct methanol fuel cells. J. Memb. Sci. 2002, 201, 47–54. [Google Scholar] [CrossRef]
- Tang, H.; Pintauro, P.N. Polyphosphazene membranes. IV. Polymer morphology and proton conductivity in sulfonated poly[bis(3-methylphenoxy)phosphazene] films. J. Appl. Polym. Sci. 2000, 79, 49–59. [Google Scholar] [CrossRef]
- Jones, D.J.; Roziere, J. Recent advances in the functionalization of polybenzimidazole and polyetherketone for fuel cell applications. J. Memb. Sci. 2001, 185, 41–58. [Google Scholar] [CrossRef]
- Genova-Dimitrova, P.; Baradie, B.; Foscallo, D.; Poinsignon, C.; Sanchez, J.Y. Ionomeric membranes for proton exchange membrane fuel cell (PEMFC): Sulfonated polysulfone associated with phosphatoantimonic acid. J. Memb. Sci. 2001, 185, 59–71. [Google Scholar] [CrossRef]
- Lufrano, F.; Squadrito, G.; Patti, A.; Passalacqua, E. Sulfonated polysulfone as promising membranes for polymer electrolyte fuel cells. J. Appl. Polym. Sci. 2000, 77, 1250–1256. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.K.; Lee, H.S.; Lane, O.; Moore, R.B.; McGrath, J.E.; Baird, D.G. Effects of block length and solution-casting conditions on the final morphology and properties of disulfonated poly(arylene ether sulfone) multiblock copolymer films for proton exchange membranes. Polymer 2009, 50, 6129–6138. [Google Scholar] [CrossRef]
- Wang, C.; Shin, D.W.; Lee, S.Y.; Kang, N.R.; Lee, Y.M.; Guiver, M.D. Poly(arylene ether sulfone) proton exchange membranes with flexible acid side chains. J. Memb. Sci. 2012, 405, 68–78. [Google Scholar] [CrossRef]
- Seo, D.W.; Lim, Y.D.; Lee, S.H.; Hossain, M.A.; Islam, M.M.; Lee, H.C.; Jang, H.H.; Kim, W.G. Preparation and characterization of block copolymers containing multi-sulfonated units for proton exchange membrane fuel cells. Electrochimica. Acta. 2012, 86, 352–359. [Google Scholar] [CrossRef]
- Erce, S.; Erdener, H.; Akay, R.G.; Yuecel, H.; Bac, N.; Eroglu, I. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrog. Energ. 2009, 34, 4645–4652. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric proton conducting membranes for medium temperature fuel cells (110–160 °C). J. Memb. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Rowlett, J.R.; Chen, Y.; Shaver, A.T.; Lane, O.; Mittelsteadt, C.; Xu, H.; Zhang, M.; Moore, R.B.; Mecham, S.; McGrath, J.E. Multiblock poly(arylene ether nitrile) disulfonated poly(arylene ether sulfone) copolymers for proton exchange membranes: Part 1 synthesis and characterization. Polymer 2013, 54, 6305–6313. [Google Scholar] [CrossRef]
- Shin, D.W.; Lee, S.Y.; Lee, C.H.; Lee, K.S.; Park, C.H.; McGrath, J.E.; Zhang, M.; Moore, R.B.; Lingwood, M.D.; Madsen, L.A.; et al. Sulfonated poly(arylene sulfide sulfone nitrile) multiblock copolymers with ordered morphology for proton exchange membranes. Macromolecules 2013, 46, 7797–7804. [Google Scholar] [CrossRef]
- Junpei, M.; Masaki, S.; Ryo, A.; Masahiro, W.; Kenji, M. A proton conductive aromatic block copolymer containing dibenzofuran moieties. Chem. Lett. 2015, 44, 964–966. [Google Scholar]
- Masanori, H.; Masaya, H.; Kenji, M.; Junji, I.; Masahiro, W. Effects of hot liquid-water treatment on local proton conductivity at surfaces of sulfonated poly(arylene ketone) block copolymer membrane for fuel cells studied by current-sensing atomic force microscopy. Eletrochimica Acta 2014, 143, 383–389. [Google Scholar]
- Lee, H.S.; Roy, A.; Lane, O.; Lee, M.B.; McGrath, J.E. Synthesis and characterization of multiblock copolymers based on hydrophilic disulfonated poly(arylene ether sulfone) and hydrophobic partially fluorinated poly(arylene ether ketone) for fuel cell applications. J. Polym. Sci. Part A 2010, 48, 214–222. [Google Scholar] [CrossRef]
- Li, S.; Tomoya, H.; Rina, M.; Teruaki, H. Mitsuru Ueda. Block copolystyrene derivatives having flexible alkylsulfonated side chains and hydrophobic alkoxy chains as a proton exchange membrane for fuel cell application. J. Poly. Sci. Part A 2013, 51, 2216–2224. [Google Scholar]
- Fujimoto, C.H.; Hickner, M.A.; Cornelius, C.J.; Loy, D.A. Ionomeric poly(phenylene) prepared by Diels-Alder polymerization: Synthesis and physical properties of a novel polyelectrolyte. Macromolecules 2005, 38, 5010–5016. [Google Scholar] [CrossRef]
- Guo, C.; Budy, S.M.; Loy, D.A. Asymmetric membranes by wet phase inversion of phenylated polyphenylene. J. Appl. Polym. Sci. 2013, 128, 750–753. [Google Scholar] [CrossRef]
- Maria, T.G.; Daniel, R.N.; Serguei, F.; Salvador, L.M.; Mikhail, G.Z.; Hernandez, M.C.; Kricheldorf, H.; Edward, S.W. Dramatic enhancement of superacid-catalyzed polyhydroxyalkylation reactions. Macromolecules 2011, 44, 194–202. [Google Scholar]
- Lancucki, L.; Schlick, S.; Danilczuk, M.; Coms, F.D.; Kruczala, K. Sulfonated poly(benzoyl paraphenylene) as a membrane for PEMFC: Ex situ and in situ experiments of thermal and chemical stability. Polym. Degrad. Stab. 2013, 98, 3–11. [Google Scholar] [CrossRef]
- Wu, S.; Qiu, Z.; Zhang, S.; Yang, X.; Yang, F.; Li, Z. The direct synthesis of wholly aromatic poly(p-phenylene)s bearing sulfobenzoyl side groups as proton exchange membranes. Polymer 2006, 47, 6993–7000. [Google Scholar] [CrossRef]
- Ghassemi, H.; McGrath, J.E. Synthesis and properties of new sulfonated poly(p-phenylene) derivatives for proton exchange membranes. I. Polymer 2004, 45, 5847–5854. [Google Scholar] [CrossRef]
- Ueda, M.; Ichikawa, F. Synthesis of aromatic poly(ether ketone)s by nickel-catalyzed coupling polymerization of aromatic dichlorides. Macromolecules 1990, 23, 926–930. [Google Scholar] [CrossRef]
- Lim, Y.D.; Lee, H.C.; Lee, S.H.; Jang, H.H.; Hossain, M.A.; Cho, Y.G.; Kim, T.H.; Hong, Y.T.; Kim, W.G. Synthesis and properties of sulfonated poly(phenylene sulfone)swithout ether linkage by Diels–Alder reaction for PEMFC application. Electrochimica Acta 2014, 119, 16–23. [Google Scholar] [CrossRef]
- Jang, H.H.; Hong, T.H.; Yoo, J.H.; Lee, S.H.; Pyo, J.S.; Sutradhar, S.C.; Ju, H.C.; Kim, W.G. Preparation and characterization of sulfonated poly(phenylene)s membranes containing conjugated moiety via nickel catalyzed carbon-carbon coupling polymerization. Int. J. Hydrog. Energy 2015, 40, 14364–14370. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, Y.D.; Hossain, M.A.; Jang, H.H.; Jeon, Y.T.; Lee, S.Y.; Jin, L.; Kim, W.G. Synthesis and properties of grafting sulfonated polymer containing isatin by super acid-catalyzed polyhydroxyalkylation reaction for PEMFC. Renew. Energy 2015, 79, 72–77. [Google Scholar] [CrossRef]
- Yin, Y.; Fang, J.; Kita, H.; Okamoto, K.I. Novel sulfoalkoxyated polyimide membrane for polymer electrolyte fuel cells. Chem. Lett. 2003, 32, 328–329. [Google Scholar] [CrossRef]
- Lim, Y.D.; Lee, S.H.; Jang, H.H.; Hossain, M.A.; Choi, S.Y.; Cho, Y.G.; Lim, J.S.; Kim, W.G. Synthesis and characterization of pendant propane sulfonic acid on phenylene based copolymers by superacid-catalyzed reaction. Renew. Energy 2015, 79, 85–90. [Google Scholar] [CrossRef]











| Polymer | SPIES | GSPIES | Nafion 211® |
|---|---|---|---|
| Theoretical IEC (meq./g) | 1.49 | 1.40 | - |
| Titrated IEC (meq./g) | 1.46 | 0.71 | 0.91 |
| Water uptake a (%) | 69.3 | 9.12 | 32.1 |
| Δt b (%) | 22.0 | 8.1 | 14.1 |
| Δl b (%) | 10.2 | 5.0 | 13.8 |
| Young‘s modulus c (Mpa) | 1,169 | 1,185 | 208 |
| Proton conductivity d (mS/cm) | 65.4 | 47.2 | 104.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Jang, H.; Yoo, J.; Ha, J.; Choi, K.; Ryu, T.; Lee, S.; Kim, W. Studies of Grafted and Sulfonated Spiro Poly(isatin-ethersulfone) Membranes by Super Acid-Catalyzed Reaction. Polymers 2016, 8, 114. https://doi.org/10.3390/polym8040114
Jin L, Jang H, Yoo J, Ha J, Choi K, Ryu T, Lee S, Kim W. Studies of Grafted and Sulfonated Spiro Poly(isatin-ethersulfone) Membranes by Super Acid-Catalyzed Reaction. Polymers. 2016; 8(4):114. https://doi.org/10.3390/polym8040114
Chicago/Turabian StyleJin, Lei, Hohyoun Jang, Jiho Yoo, Jaeseong Ha, Kunyoung Choi, Taewook Ryu, Sungkwun Lee, and Whangi Kim. 2016. "Studies of Grafted and Sulfonated Spiro Poly(isatin-ethersulfone) Membranes by Super Acid-Catalyzed Reaction" Polymers 8, no. 4: 114. https://doi.org/10.3390/polym8040114
APA StyleJin, L., Jang, H., Yoo, J., Ha, J., Choi, K., Ryu, T., Lee, S., & Kim, W. (2016). Studies of Grafted and Sulfonated Spiro Poly(isatin-ethersulfone) Membranes by Super Acid-Catalyzed Reaction. Polymers, 8(4), 114. https://doi.org/10.3390/polym8040114