Recent Advances in Nanostructured Conducting Polymers: from Synthesis to Practical Applications
Abstract
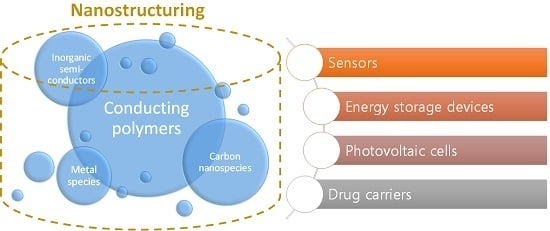
:1. Introduction
2. Overview and Classification of Synthesis Methods
2.1. Polymerization Mechanism
2.2. Methods for Fabricating Conducting Polymer Nanomaterials
2.2.1. Solid Template Approach
2.2.2. Molecular Template Approach
2.2.3. Template-Free Approach
2.2.4. Other Methods
2.3. Synthesis Methods for CP Nanohybrids
3. Progress in CP Nanomaterial Synthesis
3.1. Recent Advances in the Synthesis of CP Nanomaterials
3.2. Novel Trends for Synthesis of CP Nanohybrids
4. Selected Applications of CP Nanomaterials
4.1. Sensors
4.2. Electrochemical Energy Storage Devices
4.3. Photovoltaic Cells
4.4. Drug Carriers
5. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.Z.; Li, M.M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, Q. Controlled synthesis and energy applications of one-dimensional conducting polymer nanostructures: An overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef]
- Janáky, C.; Rajeshwar, K. The role of (photo)electrochemistry in the rational design of hybrid conducting polymer/semiconductor assemblies: From fundamental concepts to practical applications. Prog. Polym. Sci. 2015, 43, 96–135. [Google Scholar] [CrossRef]
- Park, S.; Kwon, O.; Lee, J.; Jang, J.; Yoon, H. Conducting polymer-based nanohybrid transducers: A potential route to high sensitivity and selectivity sensors. Sensors 2014, 14, 3604–3630. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Choi, M.; Lee, K.J.; Jang, J. Versatile strategies for fabricating polymer nanomaterials with controlled size and morphology. Macromol. Res. 2008, 16, 85–102. [Google Scholar] [CrossRef]
- Yoon, H.; Jang, J. Conducting-polymer nanomaterials for high-performance sensor applications: Issues and challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Guo, J.; Liu, P. Effect of the oxidant/monomer ratio and the washing post- treatment on electrochemical properties of conductive polymers. Ind. Eng. Chem. Res. 2014, 53, 13680–13689. [Google Scholar] [CrossRef]
- Tantawy, H.R.; Weakley, A.T.; Aston, D.E. Chemical effects of a solvent-limited approach to HCl-doped polyaniline nanopowder synthesis. J. Phys. Chem. C 2013, 118, 1294–1305. [Google Scholar] [CrossRef]
- Mu, J.; Ma, G.; Peng, H.; Li, J.; Sun, K.; Lei, Z. Facile fabrication of self-assembled polyaniline nanotubes doped with d-tartaric acid for high-performance supercapacitors. J. Power Sources 2013, 242, 797–802. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, Q.; Zhang, H.; Li, J.; Wang, X.; Wang, F. Water dispersed conducting polyaniline nanofibers for high-capacity rechargeable lithium—oxygen battery. ACS Macro Lett. 2013, 2, 92–95. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Feng, T.; Han, R.; Liu, D.; He, T. Preparation of polypyrrole thin film counter electrode with pre-stored iodine and resultant influence on its performance. J. Power Sources 2015, 274, 1076–1084. [Google Scholar] [CrossRef]
- Severt, S.Y.; Ostrovsky-Snider, N.A.; Leger, J.M.; Murphy, A.R. Versatile method for producing 2D and 3D conductive biomaterial composites using sequential chemical and electrochemical polymerization. ACS Appl. Mater. Interfaces 2015, 7, 25281–25288. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Lee, S.M.; Park, N., II; Lee, S.; Chung, D. Preparation and characterization of conducting polymer nanocomposite with partially reduced graphene oxide. Synth. Met. 2015, 201, 61–66. [Google Scholar] [CrossRef]
- Feng, W.; Wan, A.S.; Garfunkel, E. Interfacial bonding and morphological control of electropolymerized polythiophene films on ZnO. J. Phys. Chem. C 2013, 117, 9852–9863. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Lu, S.; Su, J.; He, T. Influence of doping anions on structure and properties of electro-polymerized polypyrrole counter electrodes for use in dye-sensitized solar cells. J. Power Sources 2014, 246, 491–498. [Google Scholar] [CrossRef]
- Chen, S.; Zhitomirsky, I. Polypyrrole electrodes doped with sulfanilic acid azochromotrop for electrochemical supercapacitors. J. Power Sources 2013, 243, 865–871. [Google Scholar] [CrossRef]
- McGarry, S.P.; Barrera Ramirez, E.A.; Tarr, N.G. Modelling electrochemical control of percolation conductivity in short-chain templated conducting polymers. Synth. Met. 2015, 200, 156–163. [Google Scholar] [CrossRef]
- Sangermano, M.; Sordo, F.; Chiolerio, A.; Yagci, Y. One-pot photoinduced synthesis of conductive polythiophene-epoxy network films. Polymer 2013, 54, 2077–2080. [Google Scholar] [CrossRef]
- Yamada, K.; Yamada, Y.; Sone, J. Three-dimensional photochemical microfabrication of poly(3,4-ethylene-dioxythiophene) in transparent polymer sheet. Thin Solid Films 2014, 554, 102–105. [Google Scholar] [CrossRef]
- Janáky, C.; Chanmanee, W.; Rajeshwar, K. Mechanistic aspects of photoelectrochemical polymerization of polypyrrole on a TiO2 nanotube array. Electrochim. Acta 2014, 122, 303–309. [Google Scholar] [CrossRef]
- Ngaboyamahina, E.; Cachet, H.; Pailleret, A.; Sutter, E.M.M. Photo-assisted electrodeposition of an electrochemically active polypyrrole layer on anatase type titanium dioxide nanotube arrays. Electrochim. Acta 2014, 129, 211–221. [Google Scholar] [CrossRef]
- Su, P.G.; Peng, Y.T. Fabrication of a room-temperature H2S gas sensor based on PPy/WO3 nanocomposite films by in-situ photopolymerization. Sen. Actuators B Chem. 2014, 193, 637–643. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Polym. Biomed. Appl. 2007, 32, 876–921. [Google Scholar]
- Terje, A.; Skotheim, J.R.R. Conjugated Polymers Theory, Synthesis, Properties, and Characterization; CRC Press: Boca Raton, FL, USA, 1993; Volume 1. [Google Scholar]
- Babu, K.F.; Senthilkumar, R.; Noel, M.; Kulandainathan, M.A. Polypyrrole microstructure deposited by chemical and electrochemical methods on cotton fabrics. Synth. Met. 2009, 159, 1353–1358. [Google Scholar] [CrossRef]
- Sezer, E.; Ustamehmetoglu, B. Chemical and electrochemical polymerisation of pyrrole in the presence of N-substituted carbazoles. Synth. Met. 1999, 107, 7–17. [Google Scholar] [CrossRef]
- Gorey, B.; Smyth, M.R.; White, B.; Morrin, A. Fabrication of homogenous three dimensionally ordered conducting polymer–polystyrene opal structures in microfluidic channels. J. Mater. Chem. C 2014, 2, 6004–6009. [Google Scholar] [CrossRef]
- Mazzotta, E.; Surdo, S.; Malitesta, C.; Barillaro, G. High-aspect-ratio conducting polymer microtube synthesis by light-activated electropolymerization on microstructured silicon. Electrochem. Commun. 2013, 35, 12–16. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Martínez-Gutiérrez, H.; Corona-Rivera, M.A.; Cervantes-González, E.; Flores-Mejía, J.; Farías-Cepeda, L. Silver/silver bromide/polypyrrole nanoparticles obtained by microemulsion photopolymerization in the presence of a cationic surfactant. Colloid Polym. Sci. 2013, 291, 2131–2138. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Amal, I.; Lee, J.; Cho, M.H. pTSA doped conducting graphene/polyaniline nanocomposite fibers: Thermoelectric behavior and electrode analysis. Chem. Eng. J. 2014, 242, 155–161. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, K.; Chen, C.; Zhang, W.; Sun, H.; Zhang, G.; Gao, Z. Structural-controlled synthesis of polyaniline nanoarchitectures using hydrothermal method. High Perform. Polym. 2014, 27, 207–216. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M.; Šeděnková, I.; Kovářová, J.; Kopecká, J.; Prokeš, J. Coaxial conducting polymer nanotubes: Polypyrrole nanotubes coated with polyaniline or poly(p-phenylenediamine) and products of their carbonisation. Chem. Pap. 2015, 69, 1341–1349. [Google Scholar] [CrossRef]
- Varga, M.; Kopecká, J.; Morávková, Z.; Křivka, I.; Trchová, M.; Stejskal, J.; Prokeš, J. Effect of oxidant on electronic transport in polypyrrole nanotubes synthesized in the presence of methyl orange. J. Polym. Sci. B Polym. Phys. 2015, 53, 1147–1159. [Google Scholar] [CrossRef]
- Dutt, S.; Siril, P.F. Controlling the morphology of polyaniline-platinum nanocomposites using swollen liquid crystal templates. Synth. Met. 2015, 209, 82–90. [Google Scholar] [CrossRef]
- Qiu, Y.; Lu, S.; Wang, S.; Zhang, X.; He, S.; He, T. High-performance polyaniline counter electrode electropolymerized in presence of sodium dodecyl sulfate for dye-sensitized solar cells. J. Power Sources 2014, 253, 300–304. [Google Scholar] [CrossRef]
- Zhou, H.; Han, G.; Fu, D.; Chang, Y.; Xiao, Y.; Zhai, H.J. Petal-shaped poly(3,4-ethylenedioxythiophene)/sodium dodecyl sulfate-graphene oxide intercalation composites for high-performance electrochemical energy storage. J. Power Sources 2014, 272, 203–210. [Google Scholar] [CrossRef]
- Oh, J.Y.; Shin, M.; Lee, J.B.; Ahn, J.H.; Baik, H.K.; Jeong, U. Effect of PEDOT nanofibril networks on the conductivity, flexibility, and coatability of PEDOT:PSS films. ACS Appl. Mater. Interfaces 2014, 6, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, H.; Jiang, H.; Zhang, K.; Chang, K.; Jia, S.; Jiang, W.; Shang, Y.; Lu, W.; Deng, S.; et al. Polypyrrole nanoparticles fabricated via Triton X-100 micelles template approach and their acetone gas sensing property. Appl. Surf. Sci. 2013, 280, 212–218. [Google Scholar] [CrossRef]
- Wan, M. A template-free method towards conducting polymer nanostructures. Adv. Mater. 2008, 20, 2926–2932. [Google Scholar] [CrossRef]
- Charlot, B.; Sassine, G.; Garraud, A.; Sorli, B.; Giani, A.; Combette, P. Micropatterning PEDOT:PSS layers. Microsyst. Technol. 2013, 19, 895–903. [Google Scholar] [CrossRef]
- Kannan, B.; Williams, D.E.; Laslau, C.; Travas-Sejdic, J. The electrochemical growth of highly conductive single PEDOT (conducting polymer):BMIPF6 (ionic liquid) nanowires. J. Mater. Chem. 2012, 22, 18132–18135. [Google Scholar] [CrossRef]
- Cardenas, J.R.; de Franca, M.G.O.; de Vasconcelos, E.A.; de Azevedo, W.M.; da Silva, E.F., Jr. Growth of sub-micron fibres of pure polyaniline using the electrospinning technique. J. Phys. D Appl. Phys. 40 2007, 40, 1068–1071. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, K.J.; Lee, Y.; Noh, S.; Yoon, H. Free-Standing, multilayered graphene/polyaniline-glue/graphene nanostructures for flexible, solid-state electrochemical capacitor application. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Wang, A.; Xu, H.; Feng, J.; Ding, L.; Tong, Y.; Li, G. Design of Pd/PANI/Pd sandwich-structured nanotube array catalysts with special shape effects and synergistic e ff ects for ethanol electrooxidation. J. Am. Chem. Soc. 2013, 135, 10703–10709. [Google Scholar] [CrossRef] [PubMed]
- Guzelturk, B.; Menk, F.; Philipps, K.; Kelestemur, Y.; Olutas, M.; Zentel, R.; Demir, H.V. Colloidal nanoplatelet/conducting polymer hybrids: excitonic and material properties. J. Phys. Chem. C 2016, 120, 3573–3582. [Google Scholar] [CrossRef]
- Rawolle, M.; Sarkar, K.; Niedermeier, M.A.; Schindler, M.; Lellig, P.; Gutmann, J.S.; Moulin, J.F.; Haese-Seiller, M.; Wochnik, A.S.; Scheu, C.; et al. Infiltration of polymer hole-conductor into mesoporous titania structures for solid-state dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 719–729. [Google Scholar] [CrossRef] [PubMed]
- We, J.H.; Kim, S.J.; Cho, B.J. Hybrid composite of screen-printed inorganic thermoelectric film and organic conducting polymer for flexible thermoelectric power generator. Energy 2014, 73, 506–512. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, X.; Zhang, Y.; Chen, L.; Liu, Z. Microspherical polyaniline/graphene nanocomposites for high performance supercapacitors. J. Power Sources 2013, 243, 715–720. [Google Scholar] [CrossRef]
- Bahloul, A.; Nessark, B.; Briot, E.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Polypyrrole-covered MnO2 as electrode material for supercapacitor. J. Power Sources 2013, 240, 267–272. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, H.; Liu, Q.; Sun, L.; Stanciu, L.; Xie, J. Fabrication of high-surface-area graphene/polyaniline nanocomposites and their application in supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, N.; Poyraz, S.; Wang, X.; Yu, Y.; Scott, J.; Smith, J.; Kim, M.J.; Zhang, X. One-pot formation of multifunctional Pt-conducting polymer intercalated nanostructures. Nanoscale 2013, 5, 3872–3879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Han, G.; Chang, Y.; Fu, D.; Xiao, Y. Highly stable multi-wall carbon nanotubes@poly(3,4-ethylenedioxythiophene)/poly(styrene sulfonate) core-shell composites with three-dimensional porous nano-network for electrochemical capacitors. J. Power Sources 2015, 274, 229–236. [Google Scholar] [CrossRef]
- Feng, Z.L.; Yao, Y.Y.; Xu, J.K.; Zhang, L.; Wang, Z.F.; Wen, Y.P. One-step co-electrodeposition of graphene oxide doped poly(hydroxymethylated-3,4-ethylenedioxythiophene) film and its electrochemical studies of indole-3-acetic acid. Chin. Chem. Lett. 2014, 25, 511–516. [Google Scholar] [CrossRef]
- Xia, X.; Chao, D.; Qi, X.; Xiong, Q.; Zhang, Y.; Tu, J.; Zhang, H.; Fan, H.J. Controllable growth of conducting polymers shell for constructing high-quality organic/inorganic core/shell nanostructures and their optical-electrochemical properties. Nano Lett. 2013, 13, 4562–4568. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Ohlan, A.; Pham, V.H.; Balasubramaniyan, R.; Varshney, S.; Jang, J.; Hur, S.H.; Choi, W.M.; Kumar, M.; Dhawan, S.K.; et al. Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution. Nanoscale 2013, 5, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Su, N.; Zhang, X.; Zhao, J.; Li, H.; Liu, X.; Zhang, H. Controllable synthesis and characterization of poly(aniline-co-pyrrole) using anionic spherical polyelectrolyte brushes as dopant and template. Polym. Compos. 2014, 35, 1858–1863. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Huang, X.; Huang, C. Synthesis and characterization of polypyrrole using TiO2 nanotube@poly(sodium styrene sulfonate) as dopant and template. Polym. Polym. Compos. 2014, 37, 462–467. [Google Scholar] [CrossRef]
- Komiyama, H.; Komura, M.; Akimoto, Y.; Kamata, K.; Iyoda, T. Longitudinal and lateral integration of conducting polymer nanowire arrays via block-copolymer-templated electropolymerization. Chem. Mater. 2015, 27, 4972–4982. [Google Scholar] [CrossRef]
- Choi, W.M. Simple and rapid fabrication of large-area 2D colloidal crystals for nanopatterning of conducting polymers. Microelectron. Eng. 2013, 110, 1–5. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Pernites, R.B.; Foster, E.L.; Advincula, R.C. Conducting polymer-gold co-patterned surfaces via nanosphere lithography. J. Colloid Interface Sci. 2015, 459, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Cernat, A.; le Goff, A.; Holzinger, M.; Sandulescu, R.; Cosnier, S. Micro- to nanostructured poly(pyrrole-nitrilotriacetic acid) films via nanosphere templates: Applications to 3D enzyme attachment by affinity interactions. Anal. Bioanal. Chem. 2014, 406, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Atobe, M.; Yoshida, N.; Sakamoto, K.; Sugino, K.; Fuchigami, T. Preparation of highly aligned arrays of conducting polymer nanowires using templated electropolymerization in supercritical fluids. Electrochim. Acta 2013, 87, 409–415. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nakabayashi, K.; Fuchigami, T.; Atobe, M. Electrochemical and photoelectrochemical behaviors of polythiophene nanowires prepared by templated electrodeposition in supercritical fluids. Electrochem. Soc. Japan 2013, 81, 328–330. [Google Scholar] [CrossRef]
- Kang, M.; Lee, J.E.; Shim, H.W.; Jeong, M.S.; Im, W.B.; Yoon, H. Intrinsically conductive polymer binders for electrochemical capacitor application. RSC Adv. 2014, 4, 27939–27945. [Google Scholar] [CrossRef]
- Ahn, K.J.; Lee, Y.; Choi, H.; Kim, M.S.; Im, K.; Noh, S.; Yoon, H. Surfactant-templated synthesis of polypyrrole nanocages as redox mediators for efficient energy storage. Sci. Rep. 2015, 5, 14097. [Google Scholar] [CrossRef] [PubMed]
- Devaki, S.J.; Sadanandhan, N.K.; Sasi, R.; Adler, H.J.P.; Pich, A. Water dispersible electrically conductive poly(3,4-ethylenedioxythiophene) nanospindles by liquid crystalline template assisted polymerization. J. Mater. Chem. C 2014, 2, 6991–7000. [Google Scholar] [CrossRef]
- Sadanandhan, N.K.; Devaki, S.J.; Narayanan, R.K.; Cheriyathuchenaaramvalli, M. Electrochemically patterned transducer with anisotropic PEDOT through liquid crystalline template polymerization. ACS Appl. Mater. Interfaces 2015, 7, 18028–18037. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Z.; Nie, G.; Wang, C.; Lu, X. Fabrication of conducting polymer/noble metal composite nanorings and their enhanced catalytic properties. J. Mater. Chem. A 2014, 3, 83–86. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Hu, S.; Zhang, X.; Zhai, J.; Han, H.; Xiao, D.; Liu, P. Control of morphology and electromagnetic properties of polypyrrole synthesized by the template method. High Perform. Polym. 2015, 28, 255–260. [Google Scholar] [CrossRef]
- MacNeill, C.M.; Graham, E.G.; Levi-Polyachenko, N.H. Soft template synthesis of donor-acceptor conjugated polymer nanoparticles: Structural effects, stability, and photothermal studies. J. Polym. Sci. A Polym. Chem. 2014, 52, 1622–1632. [Google Scholar] [CrossRef]
- Sharma, M.; Waterhouse, G.I.N.; Loader, S.W.C.; Garg, S.; Svirskis, D. High surface area polypyrrole scaffolds for tunable drug delivery. Int. J. Pharm. 2013, 443, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Rivero, O.; Huerta, F.; Montilla, F.; Sanchis, C.; Morallón, E. Electrocatalytic oxidation of ascorbic acid on mesostructured SiO2-conducting polymer composites. Eur. Polym. J. 2015, 69, 201–207. [Google Scholar] [CrossRef]
- Samu, G.F.; Visy, C.; Rajeshwar, K.; Sarker, S.; Subramanian, V.R.; Janáky, C. Photoelectrochemical infiltration of a conducting polymer (PEDOT) into metal-chalcogenide decorated TiO2 nanotube arrays. Electrochim. Acta 2015, 151, 467–476. [Google Scholar] [CrossRef]
- Sukchol, K.; Thongyai, S.; Praserthdam, P.; Sotzing, G.A. Effects of the addition of anionic surfactant during template polymerization of conducting polymers containing PEDOT with sulfonated poly(imide) and poly(styrene sulfonate) as templates for nano-thin film applications. Synth. Met. 2013, 179, 10–17. [Google Scholar] [CrossRef]
- Liao, J.; Wu, S.; Yin, Z.; Huang, S.; Ning, C.; Tan, G.; Chu, P.K. Surface-dependent self-assembly of conducting polypyrrole nanotube arrays in template-free electrochemical polymerization. ACS Appl. Mater. Interfaces 2014, 6, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Fumagalli, L.; Teixidor, F.; Samitier, J.; Errachid, A. Directing polypyrrole growth by chemical micropatterns: A study of high-throughput well-ordered arrays of conductive 3D microrings. Sens. Actuators B Chem. 2013, 177, 1003–1009. [Google Scholar] [CrossRef]
- Kong, S.; Fontaine, O.; Roche, J.; Bouffier, L.; Kuhn, A.; Zigah, D. Electropolymerization of polypyrrole by bipolar electrochemistry in an ionic liquid. Langmuir 2014, 30, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Sato, K.; Kondo, M.; Shimomura, M. Targeted deposition of a conducting polymer based on bipolar electrochemistry. Synth. Met. 2014, 198, 274–276. [Google Scholar] [CrossRef]
- Junker, K.; Zandomeneghi, G.; Schuler, L.D.; Kissner, R.; Walde, P. Enzymatic polymerization of pyrrole with Trametes versicolor laccase and dioxygen in the presence of vesicles formed from AOT (sodium bis-(2-ethylhexyl) sulfosuccinate) as templates. Synth. Met. 2015, 200, 123–134. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, D.; He, F.; Ni, C.; Chi, L. Fabricating sub-100nm conducting polymer nanowires by edge nanoimprint lithography. J. Colloid Interface Sci. 2015, 458, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Lifincev, I.; Welland, M.E. Conducting core–shell nanowires by amyloid nanofiber templated polymerization. Biomacromolecules 2015, 16, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Liu, Q.; Ding, B. Shape-controlled nanofabrication of conducting polymer on planar DNA templates. Chem. Mater. 2014, 26, 3364–3367. [Google Scholar] [CrossRef]
- Miao, Y.E.; Fan, W.; Chen, D.; Liu, T.X. High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Kwon, J.H.; Kim, M. Highly oriented self-assembly of conducting polymer chains: Extended-chain crystallization during long-range polymerization. J. Phys. Chem. C 2013, 117, 15402–15408. [Google Scholar] [CrossRef]
- Li, J.; Yoon, S.J.; Hsieh, B.Y.; Tai, W.; O’Donnell, M.; Gao, X. Stably doped conducting polymer nanoshells by surface initiated polymerization. Nano Lett. 2015, 15, 8217–8222. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, Z.; Song, N.; Zhang, Y.; Yang, Y.; Wang, J. Synthesis of hollow polyaniline nano-capsules and their supercapacitor application. J. Power Sources 2014, 272, 915–921. [Google Scholar] [CrossRef]
- Wannapob, R.; Vagin, M.Y.; Jeerapan, I.; Mak, W.C. Pure nanoscale morphology effect enhancing the energy storage characteristics of processable hierarchical polypyrrole. Langmuir 2015, 31, 11904–11913. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, Y.; Wang, J.; Gao, M.; Wang, J. Interface effect on the electropolymerized polypyrrole films with hollow micro/nanohorn arrays. ACS Appl. Mater. Interfaces 2014, 6, 4693–4704. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; Schaming, D.; Martin, P.; Lacroix, J.C. Highly resolved nanostructured PEDOT on large areas by nanosphere lithography and electrodeposition. ACS Appl. Mater. Interfaces 2015, 7, 21673–21681. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Zou, J.; Chen, X.; Munshi, A.; Smith, N.M.; Agarwal, V.; Hodgetts, S.I.; Plant, G.W.; Bakker, A.J.; Harvey, A.R.; Luzinov, I.; Iyer, K.S. Hierarchical patterning of multifunctional conducting polymer nanoparticles as a bionic platform for topographic contact guidance. ACS Nano 2015, 9, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Cui, Z.; Archirel, P.; Pernot, P.; Marignier, J.L.; Remita, S. Electron-induced growth mechanism of conducting polymers: A coupled experimental and computational investigation. J. Phys. Chem. B 2015, 119, 5282–5298. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Kim, T.; Huh, J.; Kang, M.; Lee, J.E.; Yoon, H. Anisotropic growth control of polyaniline nanostructures and their morphology-dependent electrochemical characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Yoon, H.; Jang, J. Kinetic study of the formation of polypyrrole nanoparticles in water-soluble polymer/metal cation systems: A light-scattering analysis. Small 2010, 6, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, S.; Jiang, L.; Sun, H.; Sun, G. Controllable synthesis of vertically aligned polypyrrole nanowires as advanced electrode support for fuel cells. J. Power Sources 2014, 256, 125–132. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Wang, G. Polyaniline nanofibers with a high specific surface area and an improved pore structure for supercapacitors. J. Power Sources 2015, 294, 16–21. [Google Scholar] [CrossRef]
- Shen, J.; Wei, M.; Busnaina, A.; Barry, C.; Mead, J. Directed assembly of conducting polymers on sub-micron templates by electrical fields. Mater. Sci. Eng. B 2013, 178, 190–201. [Google Scholar] [CrossRef]
- Bhandari, S.; Khastgir, D. Template-free solid state synthesis of ultra-long hairy polyaniline nanowire supercapacitor. Mater. Lett. 2014, 135, 202–205. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Park, H.W.; Kim, T.; Kang, M.; Jang, J.; Yoon, H. Kinetically controlled formation of multidimensional poly(3,4-ethylenedioxythiophene) nanostructures in vapor-deposition polymerization. Chem. Mater. 2012, 24, 4088–4092. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Yoon, H.; Jang, J. Highly sensitive and selective chemiresistive sensors based on multidimensional polypyrrole nanotubes. Chem. Commun. 2012, 48, 10526–10528. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jin, J.; Shu, X.; Xia, J. Solid state synthesis of poly(3,4-ethylenedioxythiophene) as counter electrode for dye-sensitized solar cell. J. Power Sources 2014, 248, 1234–1240. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, B.; Luo, S.C.; Lin, H.A.; Nakao, A.; Yamashita, Y.; Yu, H.H. Controlled protein absorption and cell adhesion on polymer-brush-grafted poly(3,4-ethylenedioxythiophene) films. ACS Appl. Mater. Interfaces 2013, 5, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Lei, Y.; Gu, L.; Xiao, D. Microwave-assisted chemical-vapor-induced in situ polymerization of polyaniline nanofibers on graphite electrode for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 19978–19989. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, Y.; Ahn, K.J.; Huh, J.; Shim, H.W.; Sampath, G.; Im, W. Bin; Huh, Y.; Yoon, H. Role of co-vapors in vapor deposition polymerization. Sci. Rep. 2015, 5, 8420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synth. Met. 2013, 163, 19–23. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Dong, W.; Chen, H.; Huang, X.; Sun, B.; Chen, L. Temperature dependent conductivity of vapor-phase polymerized PEDOT films. Synth. Met. 2013, 176, 86–91. [Google Scholar] [CrossRef]
- Cho, B.; Park, K.S.; Baek, J.; Oh, H.S.; Koo Lee, Y.E.; Sung, M.M. Single-crystal poly(3,4-ethylenedioxythiophene) nanowires with ultrahigh conductivity. Nano Lett. 2014, 14, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Vucaj, N.; Quinn, M.; Baechler, C.; Notley, S.M.; Cottis, P.; Hojati-Talemi, P.; Fabretto, M.V.; Wallace, G.G.; Evans, D.R. Vapour phase synthesis of conducting polymer nanocomposites incorporating 2D nanoparticles. Chem. Mater. 2014, 26, 4207–4213. [Google Scholar] [CrossRef]
- D’Arcy, J.M.; El-Kady, M.F.; Khine, P.P.; Zhang, L.; Lee, S.H.; Davis, N.R.; Liu, D.S.; Yeung, M.T.; Kim, S.Y.; Turner, C.L.; et al. Vapor-phase polymerization of nanofibrillar poly(3,4-ethylenedioxythiophene) for supercapacitors. ACS Nano 2014, 8, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, W.; Li, S.; Yang, X.; Xu, J.; Jiang, Y. Manganese dioxide nanoparticle enrichment in porous conducting polymer as high performance supercapacitor electrode materials. Electrochim. Acta 2015, 165, 323–329. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, X.; Liu, J.; Peng, L.; Chen, C.; Huang, Z.; Li, L.; Yu, X.; Shang, S. Assembly of polypyrrole nanotube@MnO2 composites with an improved electrochemical capacitance. Mater. Sci. Eng. B 2015, 198, 51–56. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Wang, J.; Kang, L.; Lei, Z.; Liu, Z.; Liu, Z.; Hao, Z.; Liu, Z. Adsorption-template preparation of polyanilines with different morphologies and their capacitance. Electrochim. Acta 2014, 145, 99–108. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Morphology-dependent enhancement of the pseudocapacitance of template-guided tunable polyaniline nanostructures. J. Phys. Chem. C 2013, 117, 15009–15019. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J. Mater. Chem. A 2013, 1, 3315–3324. [Google Scholar] [CrossRef]
- Bogdanović, U.; Pašti, I.; Ćirić-Marjanović, G.; Mitrić, M.; Ahrenkiel, S.P.; Vodnik, V. Interfacial synthesis of gold-polyaniline nanocomposite and its electrocatalytic application. ACS Appl. Mater. Interfaces 2015, 7, 28393–28403. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, U.; Vodnik, V.; Mitrić, M.; Dimitrijević, S.; Škapin, S.D.; Žunič, V.; Budimir, M.; Stoiljković, M. Nanomaterial with high antimicrobial efficacy—copper/polyaniline nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Tu, J.; Zhou, D.; Zhang, J.; Xiong, Q.; Zhao, X. Multicolor electrochromic film based on TiO2@ polyaniline core/shell nanorod array. J. Phys. Chem. B 2013, 117, 15967–15975. [Google Scholar] [CrossRef]
- Gulce, H.; Eskizeybek, V.; Haspulat, B.; Sari, F.; Gulce, A.; Avci, A. Preparation of a new polyaniline/CdO nanocomposite and investigation of its photocatalytic activity: comparative study under UV light and natural sunlight irradiation. Ind. Eng. Chem. Res. 2013, 52, 10924–10934. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.H. Three-dimensional tubular MoS2/PANI hybrid electrode for high rate performance supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 28294–28302. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Wang, X.; Kwak, S.K.; Lee, J. Synthesis of mesoporous polyaniline (PANI)-Se0.5Te0.5 dual-layer film from lyotropic liquid crystalline template. Ind. Eng. Chem. Res. 2013, 52, 5072–5078. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Varanasi, J.L.; Das, D.; Pradhan, D. Bifunctional manganese ferrite/polyaniline hybrid as electrode material for enhanced energy recovery in microbial fuel cell. ACS Appl. Mater. Interfaces 2015, 7, 20657–20666. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, G.; Zhang, S.; Wei, Y.; Wang, J.; Li, Q. Polypyrrole/silver coaxial nanowire independent stress sensing and stress-triggered joule heating. ACS Nano 2015, 9, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Hnida, K.E.; Socha, R.P.; Sulka, G.D. Polypyrrole-silver composite nanowire arrays by cathodic co-deposition and their electrochemical properties. J. Phys. Chem. C 2013, 117, 19382–19392. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Yang, B.; Wang, R.; Wang, T. Ultrasound assisted polymerization for synthesis of ZnO/Polypyrrole composites for zinc/nickel rechargeable battery. J. Power Sources 2014, 271, 143–151. [Google Scholar] [CrossRef]
- Yin, Z.; Fan, W.; Ding, Y.; Li, J.; Guan, L.; Zheng, Q. Shell structure control of PPy-modified CuO composite nanoleaves for lithium batteries with improved cyclic performance. ACS Sustain. Chem. Eng. 2015, 3, 507–517. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Li, Y.; Liu, J. Construction of high-capacitance 3D CoO@polypyrrole nanowire array electrode for aqueous asymmetric supercapacitor. Nano Lett. 2013, 13, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Peng, H.; Mu, J.; Huang, H.; Zhou, X.; Lei, Z. In situ intercalative polymerization of pyrrole in graphene analogue of MoS2 as advanced electrode material in supercapacitor. J. Power Sources 2013, 229, 72–78. [Google Scholar] [CrossRef]
- Ngaboyamahina, E.; Debiemme-Chouvy, C.; Pailleret, A.; Sutter, E.M.M. Electrodeposition of polypyrrole in TiO2 nanotube arrays by pulsed-light and pulsed-potential methods. J. Phys. Chem. C 2014, 118, 26341–26350. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, X.J.; Zhu, Y.S.; Hu, C.L.; Wu, Y.P.; Holze, R. Polypyrrole-coated LiV3O8-nanocomposites with good electrochemical performance as anode material for aqueous rechargeable lithium batteries. J. Power Sources 2013, 224, 290–294. [Google Scholar] [CrossRef]
- Zhong, X.B.; Wang, H.Y.; Yang, Z.Z.; Jin, B.; Jiang, Q.C. Facile synthesis of mesoporous ZnCO2O4 coated with polypyrrole as an anode material for lithium-ion batteries. J. Power Sources 2015, 296, 298–304. [Google Scholar] [CrossRef]
- Guo, C.X.; Sun, K.; Ouyang, J.; Lu, X. Layered V2O5/PEDOT nanowires and ultrathin nanobelts fabricated with a silk reelinglike process. Chem. Mater. 2015, 27, 5813–5819. [Google Scholar] [CrossRef]
- Taccola, S.; Greco, F.; Zucca, A.; Innocenti, C.; De Julián Fernández, C.; Campo, G.; Sangregorio, C.; Mazzolai, B.; Mattoli, V. Characterization of free-standing PEDOT:PSS/iron oxide nanoparticle composite thin films and application as conformable humidity sensors. ACS Appl. Mater. Interfaces 2013, 5, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.E.; Jones, S.T.; Walsh, Z.; Appel, E.A.; Abo-Hamed, E.K.; Scherman, O.A. Synthesis of conducting polymer-metal nanoparticle hybrids exploiting RAFT polymerization. ACS Macro Lett. 2015, 4, 255–259. [Google Scholar] [CrossRef]
- Attia, M.F.; Azib, T.; Salmi, Z.; Singh, A.; Decorse, P.; Battaglini, N.; Lecoq, H.; Omastová, M.; Higazy, A. A.; Elshafei, A.M.; et al. One-step UV-induced modification of cellulose fabrics by polypyrrole/silver nanocomposite films. J. Colloid Interface Sci. 2013, 393, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Salmi, Z.; Jha, P.; Joshi, N.; Kumar, A.; Decorse, P.; Lecoq, H.; Lau-Truong, S.; Aswal, D.K.; Gupta, S.K.; Chehimi, M.M. One step synthesis of highly ordered free standing flexible polypyrrole-silver nanocomposite films at air–water interface by photopolymerization. RSC Adv. 2013, 3, 13329–13336. [Google Scholar] [CrossRef]
- Heydarnezhad, H.R.; Pourabbas, B. One-step synthesis of conductive ceria/polypyrrole nanocomposite particles via photo-induced polymerization method. J. Mater. Sci. Mater. Electron. 2013, 24, 4378–4385. [Google Scholar] [CrossRef]
- Asmussen, S.; Arenas, G.; Vallo, C. Photopolymerization of pyrrole/methacrylate mixtures using α-cleavage type photoinitiators in combination with iodonium salt. Synth. Met. 2015, 209, 304–312. [Google Scholar] [CrossRef]
- Jlassi, K.; Singh, A.; Aswal, D.K.; Losno, R.; Benna-Zayani, M.; Chehimi, M.M. Novel, ternary clay/polypyrrole/silver hybrid materials through in situ photopolymerization. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 439, 193–199. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Shen, Y.; Park, B.W.; Hao, Y.; Johansson, E.M.J.; Boschloo, G.; Kloo, L.; Gabrielsson, E.; Sun, L.; et al. Poly(3,4-ethylenedioxythiophene) hole-transporting material generated by photoelectrochemical polymerization in aqueous and organic medium for all-solid-state dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 16591–16601. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Shen, Y.; Park, B.W.; Bi, D.; Häggman, L.; Johansson, E.M.J.; Boschloo, G.; Hagfeldt, A.; Vlachopoulos, N.; et al. New approach for preparation of efficient solid-state dye-sensitized solar cells by photoelectrochemical polymerization in aqueous micellar solution. J. Phys. Chem. Lett. 2013, 4, 4026–4031. [Google Scholar] [CrossRef]
- Park, B.W.; Yang, L.; Johansson, E.M.J.; Vlachopoulos, N.; Chams, A.; Perruchot, C.; Jouini, M.; Boschloo, G.; Hagfeldt, A. Neutral, polaron, and bipolaron states in PEDOT prepared by photoelectrochemical polymerization and the effect on charge generation mechanism in the solid-state dye-sensitized solar cell. J. Phys. Chem. C 2013, 117, 22484–22491. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Li, Y. Fabrication of graphene oxide/polypyrrole nanowire composite for high performance supercapacitor electrodes. J. Power Sources 2013, 241, 388–395. [Google Scholar] [CrossRef]
- Song, Y.; Xu, J.L.; Liu, X.X. Electrochemical anchoring of dual doping polypyrrole on graphene sheets partially exfoliated from graphite foil for high-performance supercapacitor electrode. J. Power Sources 2014, 249, 48–58. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Polypyrrole coated carbon nanotubes for supercapacitor devices with enhanced electrochemical performance. J. Power Sources 2014, 268, 233–239. [Google Scholar] [CrossRef]
- Wang, M.; Jamal, R.; Wang, Y.; Yang, L.; Liu, F.; Abdiryim, T. Functionalization of graphene oxide and its composite with poly(3,4-ethylenedioxythiophene) as electrode material for supercapacitors. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, H.; Kim, M.S.; Noh, S.; Ahn, K.J.; Im, K.; Kwon, O.S.; Yoon, H. Nanoparticle-mediated physical exfoliation of aqueous-phase graphene for fabrication of three-dimensionally structured hybrid electrodes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Tang, Q.; Wang, M.; Ma, C.; Yuan, S. Complexation of polyaniline and graphene for efficient counter electrodes in dye-sensitized solar cells: Enhanced charge transfer ability. J. Power Sources 2014, 256, 8–13. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z.; Wei, T.; Zhang, M.; Jing, X. Mesoporous polyaniline film on ultra-thin graphene sheets for high performance supercapacitors. J. Power Sources 2014, 247, 197–203. [Google Scholar] [CrossRef]
- Gao, S.; Zang, P.; Dang, L.; Xu, H.; Shi, F.; Liu, Z.; Lei, Z. Extraordinarily high-rate capability of polyaniline nanorod arrays on graphene nanomesh. J. Power Sources 2016, 304, 111–118. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, S.M.; Hsiao, S.T.; Liao, W.H.; Yang, S.Y.; Tien, H.W.; Ma, C.C.M.; Hu, C.C. Thickness-self-controlled synthesis of porous transparent polyaniline-reduced graphene oxide composites towards advanced bifacial dye-sensitized solar cells. J. Power Sources 2014, 260, 326–337. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Hu, N.; Yang, Z.; Wei, H.; Zhang, Y. Reduced graphene oxide/polypyrrole nanotube papers for fl exible all-solid-state supercapacitors with excellent rate capability and high energy density. J. Power Sources 2016, 302, 39–45. [Google Scholar] [CrossRef]
- Yang, X.; Liu, A.; Zhao, Y.; Lu, H.; Zhang, Y.; Wei, W.; Li, Y.; Liu, S. Three-dimensional macroporous polypyrrole-derived graphene electrode prepared by the hydrogen bubble dynamic template for supercapacitors and metal-free catalysts. ACS Appl. Mater. Interfaces 2015, 7, 23731–23740. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, H.P.; Sydlik, S.A.; Swager, T.M. Supercapacitors from free-standing polypyrrole/graphene nanocomposites. J. Phys. Chem. C 2013, 117, 10270–10276. [Google Scholar] [CrossRef]
- Kumar, G.G.; Kirubaharan, C.J.; Udhayakumar, S.; Ramachandran, K.; Karthikeyan, C.; Renganathan, R.; Nahm, K.S. Synthesis, structural, and morphological characterizations of reduced graphene oxide-supported polypyrrole anode catalysts for improved microbial fuel cell performances. ACS Sustain. Chem. Eng. 2014, 2, 2283–2290. [Google Scholar] [CrossRef]
- Li, S.; Shu, K.; Zhao, C.; Wang, C.; Guo, Z.; Wallace, G.; Liu, H.K. One-step synthesis of graphene/polypyrrole nanofiber composites as cathode material for a biocompatible zinc/polymer battery. ACS Appl. Mater. Interfaces 2014, 6, 16679–16686. [Google Scholar] [CrossRef] [PubMed]
- Bora, C.; Sharma, J.; Dolui, S. Polypyrrole/sulfonated graphene composite as electrode material for supercapacitor. J. Phys. Chem. C 2014, 118, 29688–29694. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In situ polymerization deposition of porous conducting polymer on reduced graphene oxide for gas sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.H.R.; Oliveira, M.M.; Zarbin, A.J.G. Thin and flexible all-solid supercapacitor prepared from novel single wall carbon nanotubes/polyaniline thin films obtained in liquid-liquid interfaces. J. Power Sources 2014, 260, 34–42. [Google Scholar] [CrossRef]
- Yameen, B.; Zydziak, N.; Weidner, S.M.; Bruns, M.; Barner-Kowollik, C. Conducting polymer/SWCNTs modular hybrid materials via Diels–Alder ligation. Macromolecules 2013, 46, 2606–2615. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, D.; Zhang, Q.; Goh, K.; Wei, L.; Yong, Y.; Jiang, R.; Wei, J.; Chen, Y. Ternary hybrids of amorphous nickel hydroxide-carbon nanotube-conducting polymer for supercapacitors with high energy density, excellent rate capability, and long cycle life. Adv. Funct. Mater. 2015, 25, 1063–1073. [Google Scholar] [CrossRef]
- Sekkarapatti Ramasamy, M.; Nikolakapoulou, A.; Raptis, D.; Dracopoulos, V.; Paterakis, G.; Lianos, P. Reduced graphene oxide/Polypyrrole/PEDOT composite films as efficient Pt-free counter electrode for dye-sensitized solar cells. Electrochim. Acta 2015, 173, 276–281. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Shinya, N.; Qin, L.C. Polyaniline modified graphene and carbon nanotube composite electrode for asymmetric supercapacitors of high energy density. J. Power Sources 2013, 241, 423–428. [Google Scholar] [CrossRef]
- Cui, H.F.; Du, L.; Guo, P.B.; Zhu, B.; Luong, J.H.T. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Sources 2015, 283, 46–53. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.F. High-performance porous electrodes for pseudosupercapacitors based on graphene-beaded carbon nanofibers surface-coated with nanostructured conducting polymers. J. Power Sources 2014, 262, 44–49. [Google Scholar] [CrossRef]
- Moon, S.; Jung, Y.H.; Kim, D.K. Enhanced electrochemical performance of a crosslinked polyaniline-coated graphene oxide-sulfur composite for rechargeable lithium–sulfur batteries. J. Power Sources 2015, 294, 386–392. [Google Scholar] [CrossRef]
- Pan, C.; Gu, H.; Dong, L. Synthesis and electrochemical performance of polyaniline@MnO2/graphene ternary composites for electrochemical supercapacitors. J. Power Sources 2016, 303, 175–181. [Google Scholar] [CrossRef]
- Shen, J.; Yang, C.; Li, X.; Wang, G. High-performance asymmetric supercapacitor based on nanoarchitectured polyaniline/graphene/carbon nanotube and activated graphene electrodes. ACS Appl. Mater. Interfaces 2013, 5, 8467–8476. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, Q.; Lei, W.; Xia, X.; Wang, X. Ternary nitrogen-doped graphene/nickel ferrite/polyaniline nanocomposites for high-performance supercapacitors. J. Power Sources 2014, 269, 250–259. [Google Scholar] [CrossRef]
- Hu, T.H.; Yin, Z.S.; Guo, J.W.; Wang, C. Synthesis of Fe nanoparticles on polyaniline covered carbon nanotubes for oxygen reduction reaction. J. Power Sources 2014, 272, 661–671. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, W.Y.; Chou, S.W.; Lin, T.W.; Lin, J.Y. In situ electropolymerization of polyaniline/cobalt sulfide decorated carbon nanotube composite catalyst toward triiodide reduction in dye-sensitized solar cells. J. Power Sources 2014, 266, 448–455. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, C.; Fan, Y.; Zhang, W.; Zhu, J.; Wang, X. Ternary manganese ferrite/graphene/polyaniline nanostructure with enhanced electrochemical capacitance performance. J. Power Sources 2014, 266, 384–392. [Google Scholar] [CrossRef]
- Yang, L.; Tang, Y.; Yan, D.; Liu, T.; Liu, C.; Luo, S. Polyaniline-reduced graphene oxide hybrid nanosheets with nearly vertical orientation anchoring palladium nanoparticles for highly active and stable electrocatalysis. ACS Appl. Mater. Interfaces 2015, 8, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Dhibar, S.; Das, C.K. Silver nanoparticles decorated polyaniline/multiwalled carbon nanotubes nanocomposite for high-performance supercapacitor electrode. Ind. Eng. Chem. Res. 2014, 53, 3495–3508. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, C.; Du, H.; Wang, W. Enhanced electrochemical performance of polyaniline/carbon/titanium nitride nanowire array for flexible supercapacitor. J. Power Sources 2015, 286, 561–570. [Google Scholar] [CrossRef]
- Zhao, X.; Ahn, H.J.; Kim, K.W.; Cho, K.K.; Ahn, J.H. Polyaniline-coated mesoporous carbon/sulfur composites for advanced lithium sulfur batteries. J. Phys. Chem. C 2015, 119, 7996–8003. [Google Scholar] [CrossRef]
- Peng, Y.J.; Wu, T.H.; Hsu, C.T.; Li, S.M.; Chen, M.G.; Hu, C.C. Electrochemical characteristics of the reduced graphene oxide/carbon nanotube/polypyrrole composites for aqueous asymmetric supercapacitors. J. Power Sources 2014, 272, 970–978. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Gosselink, D.; Soboleski, H.G.; Chen, P. A novel nano-sulfur/polypyrrole/graphene nanocomposite cathode with a dual-layered structure for lithium rechargeable batteries. J. Power Sources 2013, 241, 517–521. [Google Scholar] [CrossRef]
- De Oliveira, A.H.P.; de Oliveira, H.P. Carbon nanotube/ polypyrrole nanofibers core-shell composites decorated with titanium dioxide nanoparticles for supercapacitor electrodes. J. Power Sources 2014, 268, 45–49. [Google Scholar] [CrossRef]
- Lin, J.Y.; Wang, W.Y.; Chou, S.W. Flexible carbon nanotube/polypropylene composite plate decorated with poly(3,4-ethylenedioxythiophene) as efficient counter electrodes for dye-sensitized solar cells. J. Power Sources 2015, 282, 348–357. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, W.; Wang, S.; Li, Z.; Zeng, Y.; Yan, S.; Sun, Y. Simple synthesis and photoelectrochemical characterizations of polythiophene/Pd/TiO2 composite microspheres. ACS Appl. Mater. Interfaces 2014, 6, 20197–20204. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, M.; Jang, J. Screen-printable and flexible RuO2 nanoparticle-decorated PEDOT:PSS/graphene nanocomposite with enhanced electrical and electrochemical performances for high-capacity supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 10213–10227. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, J.H.; Kahng, Y.H.; Kim, N.; Kim, Y.J.; Lee, J.; Lee, T.; Lee, K. Graphene-conducting polymer hybrid transparent electrodes for efficient organic optoelectronic devices. Adv. Funct. Mater. 2014, 24, 1847–1856. [Google Scholar] [CrossRef]
- Park, S.J.; Song, H.S.; Kwon, O.S.; Chung, J.H.; Lee, S.H.; An, J.H.; Ahn, S.R.; Lee, J.E.; Yoon, H.; Park, T.H.; et al. Human dopamine receptor nanovesicles for gate-potential modulators in high-performance field-effect transistor biosensors. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Lee, J.S.; Park, E.; Kim, T.; Park, H.W.; You, S.A.; Yoon, H.; Jang, J. Multidimensional conducting polymer nanotubes for ultrasensitive chemical nerve agent sensing. Nano Lett. 2012, 12, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Xu, H.; Ding, H.; Bai, L.; Mu, Z.; Xie, Z.; Zhao, Y.; Gu, Z. Preparation of conducting polymer inverse opals and its application as ammonia sensor. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 433, 59–63. [Google Scholar] [CrossRef]
- Lee, J.E.; Shim, H.W.; Kwon, O.S.; Huh, Y.I.; Yoon, H. Real-time detection of metal ions using conjugated polymer composite papers. Analyst 2014, 139, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- La Ferrara, V.; Rametta, G.; de Maria, A. AC electric field for rapid assembly of nanostructured polyaniline onto microsized gap for sensor devices. Electrophoresis 2015, 36, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Ko, S.; Jang, J. Field-effect-transistor sensor based on enzyme-functionalized polypyrrole nanotubes for glucose detection. J. Phys. Chem. B 2008, 112, 9992–9997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, S.H.; Kwon, O.S.; Song, H.S.; Oh, E.H.; Park, T.H.; Jang, J. Polypyrrole nanotubes conjugated with human olfactory receptors: High-Performance transducers for FET-Type bioelectronic noses. Angew. Chem. 2009, 48, 2755–2758. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. A field-effect-transistor sensor based on polypyrrole nanotubes coupled with heparin for thrombin detection. Mol. Cryst. Liq. Cryst. 2008, 491, 21–31. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, J.H.; Lee, N.; Kim, B.G.; Jang, J. A novel sensor platform based on aptamer-conjugated polypyrrole nanotubes for label-free electrochemical protein detection. ChemBioChem 2008, 9, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.Y.; Lee, J.S.; You, S.A.; Yoon, H.; Park, T. H.; Jang, J. Ultrasensitive and selective recognition of peptide hormone using close-packed arrays of hPTHR-conjugated polymer nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef] [PubMed]
- Chiam, Y.S.; Lim, K.S.; Harun, S.W.; Gan, S.N.; Phang, S.W. Conducting polymer coated optical microfiber sensor for alcohol detection. Sens. Actuators A Phys. 2014, 205, 58–62. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Rakhi, R.B.; Alshareef, H.N. A general approach toward enhancement of pseudocapacitive performance of conducting polymers by redox-active electrolytes. J. Power Sources 2014, 267, 521–526. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, S.J.; Kwon, O.S.; Shim, H.W.; Jang, J.; Yoon, H. Systematic investigation on charge storage behaviour of multidimensional poly(3,4-ethylenedioxythiophene) nanostructures. RSC Adv. 2014, 4, 37529–37535. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured electrode materials for electrochemical capacitor applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef]
- Kung, C.W.; Cheng, Y.H.; Chen, H.W.; Vittal, R.; Ho, K.C. Hollow microflower arrays of PEDOT and their application for the counter electrode of a dye-sensitized solar cell. J. Mater. Chem. A 2013, 1, 10693–10702. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells—An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Yin, X.; Wu, F.; Fu, N.; Han, J.; Chen, D.; Xu, P.; He, M.; Lin, Y. Facile synthesis of poly(3,4-ethylenedioxythiophene) film via solid-state polymerization as high-performance Pt-free counter electrodes for plastic dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, W.J.; Cho, N.; Shukla, S.; Yoon, H.; Jang, J.; Prasad, P.N.; Kim, T.D.; Lee, K.S. Synthesis and properties of quantum dot-polypyrrole nanotube composites for photovoltaic application. J. Nanosci. Nanotechnol. 2009, 9, 6957–6961. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Jarosz, T.; Zak, J.K.; Lapkowski, M.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Bednarczyk-Cwynar, B. Advancing the delivery of anticancer drugs: Conjugated polymer/triterpenoid composite. Acta Biomater. 2015, 19, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.Y.; Jeon, S.H.; Lee, E.S.; Cho, Y. An integrated multifunctional platform based on biotin-doped conducting polymer nanowires for cell capture, release, and electrochemical sensing. Biomaterials 2014, 35, 9573–9580. [Google Scholar] [CrossRef] [PubMed]
- In, C.; Kim, H.; Min, B.; Choi, H. Photoinduced nonlinear mixing of terahertz dipole resonances in graphene metadevices. Adv. Mater. 2016, 28, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]















| Route | Details | Ref. |
|---|---|---|
| Chemical polymerization | Requires an oxidizing agent to synthesize the polymer. The morphology of the polymer can be controlled by varying the parameters of the process, such as monomer/oxidizing agent concentration, temperature, pH, and reaction time. | [9,10,11,12,13,14] |
| Electrochemical polymerization | An oxidizing agent is not required for this route, which is an efficient approach for depositing CPs on substrates. Some monomers are theoretically not electropolymerizable. Furthermore, it is difficult to scale up this process. A high oxidation potential may lead to over oxidation of the polymer. | [13,15,16,17,18,19] |
| Photo-polymerization | Illumination is needed for polymerization. This route was developed to solve the over oxidation problem of the electrochemical method. The process can be well controlled simply by turning the light on or off. | [20,21,22,23,24] |
| Synthesis methods | Advantages | Disadvantages |
|---|---|---|
| Solid template | Applicable to almost all CPs. Possible to precisely control the size and morphology. | A post-synthesis process is required to remove the template. Nanostructure quantity is confined by the size of the template membrane. |
| Molecular template | Relatively simple, and thus scale-up is possible under optimized conditions. | Hard to provide good uniformity of size and morphology. |
| Template-free | Simple process without templates. | Limited to certain precursors. |
| Electrospinning | Simple to produce continuous CP nanofibers. | Only soluble and thermoplastic polymers are applicable. |
| Nanoimprinting | High throughput and high resolution. | An expensive micro-mold is required. |
| Main Group | Advantage | Disadvantage | Example | Ref. |
|---|---|---|---|---|
| Ex situ synthesis | Simple Solution-processable | Limited applications Poor control of the contact between each component | Mechanical mixing | [45] |
| Layer-by-layer deposition | [46] | |||
| In situ synthesis | Variable methods based on chemical or electrochemical route. Facile control of many variables | Higher complexity as many parameters need to be considered | In situ polymerization. Electrodeposition. In situ reduction | [50,51,52] |
| One-pot synthesis | Simple Short processing time | Limited control over structure and morphology of the products | Redox reaction Co-deposition | [53,54,55] |
| CP | Polymerization method | Details | Refs. |
|---|---|---|---|
| PANI | Amyloid nanofiber template polymerization | Amyloid nanofibers were successfully used as templates for the formation of conductive core-shell nanowires | [83] |
| Planar DNA template | Production of CPs with controlled shapes on 2D polyelectrolyte templates was investigated for the first time | [84] | |
| Electrospinning using poly(amic acid) fiber as a template | Hollow nanofibers with controllable wall thicknesses were successfully obtained | [85] | |
| Dedoped chemical polymerization | Water-dispersed CP nanofibers with high capacitance were achieved by double doping | [12] | |
| Biphase interfacial polymerization | The mechanism for self-assembly in crystalline 1D nanostructures was investigated | [86] | |
| Surface-initiated polymerization | A new approach for multimodal core-shell nanoparticles with a stable doping state was reported | [87] | |
| Interfacial polymerization | A novel hollow PANI nanocapsule with holes in the wall was synthesized | [88] | |
| PPy | Time-dependent template-assisted polymerization | A new synthesis approach for the precise control of wall morphologies of colloidal microparticles was studied | [89] |
| Modified pulse potentiostatic method | A good method to control the shape of micelles at the substrate/electrolyte interface and control the morphology of CPs was proposed | [90] | |
| PEDOT | Galvanostatic electrodeposition | Good result combining a carboxylated polystyrene template made by nanosphere lithography with SDS as a molecular template was achieved | [91] |
| Non-spontaneous emulsification | A novel method using colloidal chemistry to fabricate multifunctional CPs was developed | [92] | |
| Electron pulse-enabled in situ polymerization | The mechanism of CP growth was investigated experimentally and via modeling | [93] |
| Phase | CP | Details | Refs. |
|---|---|---|---|
| Solid phase | PANI | Hairy CP nanowires were obtained via mechanochemical polymerization using citric acid as a dopant | [99] |
| PEDOT | The role of temperature in the solid-state synthesis was studied | [102] | |
| A low-cost, low-temperature method to fabricate high-performance CPs was successfully developed | [103] | ||
| Vapor phase | PANI | The effect of microwave radiation was studied | [104] |
| PPy | The role of co-vapor in the vapor-phase polymerization (VPP) method was studied | [105] | |
| An application for drug storage was carried out by depositing PPy using the chemical vapor deposition (CVD) method | [106] | ||
| PEDOT | The dependence of electrical conductivity on VPP temperature was discussed | [107] | |
| Single-crystal CP nanowires were developed using VPP with liquid-bridge-mediated nanotransfer printing | [108] | ||
| A one-step fabrication of 2D nanoparticles was investigated | [109] | ||
| The advantages of directly depositing CP nanofibers was demonstrated | [110] |
| CP | Inorganic species | Preparation method | Details | Refs. |
|---|---|---|---|---|
| PANI | Au | Interfacial polymerization | The formation mechanism of Au-PANI was presented | [116] |
| Cu | Concurrent synthesis | The structure of a Cu/PANI hybrid was studied | [117] | |
| Pd | Layer-by-layer technique | A Pd/PANI/Pd sandwich-structure nanotube array was first reported | [46] | |
| TiO2 | Combination of hydrothermal and electropolymerization | A multicolor electrochromic film was fabricated based on hybrid core-shell nanorod arrays | [118] | |
| CdO | Chemical oxidative polymerization | Aqueous diethylene glycol solution medium was used for the first time | [119] | |
| MoS2 | Vertically aligned chemical polymerization | A good example of hybrid 3D tubular structures was discussed | [120] | |
| Se0.5Te0.5 | Lyotropic liquid crystalline template | A mesoporous dual-layer film was synthesized using Brij56 surfactant | [121] | |
| MnFe2O4 | Incorporative polymerization | Dual nature of hybrid (cathode catalyst and anode modifier) was first demonstrated | [122] | |
| PPy | Ag | Incipient network conformal growth technology | A new porous material, namely, an “aero-sponge,” was proposed | [123] |
| Cathodic co-deposition | Highly stable sensing activity of the hybrid was studied | [124] | ||
| MnO2 | Electropolymerization | Effect of deposition time was reported | [51] | |
| ZnO | Ultrasound-assisted chemical polymerization | Well-controlled granular and layered nanocomposite was formed | [125] | |
| CuO | Wire template technique | The previous method was extended to study polymerization time | [126] | |
| CoO | Modified hydrothermal and post-annealing process | 3D growth of well-aligned nanowire array was developed | [127] | |
| MoS2 | In situ intercalative polymerization | A facile strategy for intercalation of PPy into MoS2 nanosheets was proposed | [128] | |
| TiO2 | Pulsed-light and pulsed-potential method | Good control of the deposition rate was demonstrated | [129] | |
| LiV3O8 | Low-temperature in situ polymerization | A new anode material for rechargeable lithium batteries was reported | [130] | |
| ZnCo2O4 | Reflux method and chemical polymerization | A facile method for fabricating mesoporous ZnCo2O4-coated PPy was developed | [131] | |
| PT | ZnO | Electropolymerization growth | Interfacial bonding and morphology control was described | [16] |
| PEDOT | V2O5 | “Cocoon-to-silk” fiber reeling method | First method for fabricating layered V2O5/PEDOT nanowires was reported | [132] |
| Iron oxide * | Spin-coated-assisted deposition with “supporting layer technique” | A new simple, fast, and inexpensive technique for the fabrication of a free-standing hybrid was reported | [133] | |
| CP ** | Ag, Au, CdSe | RAFT polymerization | Role of the direct covalent attachment was emphasized | [134] |
| Material | Preparation method | Details | Refs. |
|---|---|---|---|
| PPy/Ag | One-pot UV-induced photopolymerization | Effect of concentration ratio of composite in cellulose fabric was studied | [135] |
| One-step interfacial photopolymerization | Thin, flexible nanofilms were synthesized at the water–air interface | [136] | |
| PPy/TiO2 | Photo-assisted electrodeposition | Effect of LiClO4 in the presence of SDBS was investigated | [23] |
| Photoelectrochemical polymerization | Properties of nanohybrid in the presence of SDS was studied | [22] | |
| PPy/WO3 | In situ photopolymerization | A room-temperature H2S gas sensor was fabricated | [24] |
| PPy/ceria | Photo-induced polymerization | A good example of one-step photopolymerization was shown | [137] |
| PPy/methacrylate | UV and visible light photopolymerization | The mechanism of photopolymerization with iodonium salt was presented | [138] |
| PPy/AgBr/Ag | Microemulsion photopolymerization | Effect of different concentrations of cationic surfactant CTAB was studied | [31] |
| PT/epoxy | One-pot photoinduced synthesis | A novel methodology for fabricating a network film was given | [20] |
| Clay/PPy/Ag | In situ photopolymerization | The silanization of clay on a PPy/Ag surface was discussed | [139] |
| PEDOT/TiO2 | Photoelectrochemical polymerization | Role of donor-π-acceptor sensitizers was described | [140] |
| A new method using aqueous micellar solutions was shown | [141] | ||
| The effect of light intensity on the oxidation level of PEDOT was studied | [142] |
| Carbon | CP | Preparation method | Details | Refs. |
|---|---|---|---|---|
| GN * | PANI | In situ polymerization | A microspherical and porous structure was fabricated | [50] |
| Reflux technique | Complex of PANI and GN for enhancing charge-transfer ability was reported | [148] | ||
| Sandwiched GN–mesoporous silica as template | A novel approach to fabricate mesoporous PANI film coating on GN | [149] | ||
| Low-temperature in situ polymerization | PANI nanorods were coated on graphene nanomesh | [150] | ||
| PPy | Double-doping electropolymerization | A good example for anchoring double-doped CP on GN sheet was given | [144] | |
| PEDOT | Electrochemical codeposition | The role of SDS surfactant in the incorporation of GN into PEDOT was studied | [38] | |
| rGO | PANI | In situ reduction | A high-surface-area hybrid was reported | [52] |
| Electrostatic adsorption synthesis | The thickness was well-controlled by pH modification | [151] | ||
| PPy | Vacuum filtration method | The improvement of cycling stability was demonstrated via the addition of rGO | [152] | |
| Hydrogen bubble dynamic template | A general method for fabrication of 3D macroporous hybrid was studied | [153] | ||
| Interfacial polymerization | The comparison of two different methods was shown, emphasizing the strength of interfacial polymerization | [154] | ||
| Bioreduction technique | A new simple, environmentally benign method that was time- and cost-efficient was developed | [155] | ||
| One-step synthesis | A good example of a cathode material was shown | [156] | ||
| Interfacial polymerization | A novel electrode material was developed | [157] | ||
| PEDOT | Fast thermal treatment with in situ deposition | A good example of a hybrid CP for gas sensing was studied | [158] | |
| SWCNT | PANI | Liquid–liquid interfacial polymerization | The synthesis and characterization of hybrid thin films in liquid–liquid interface was first studied | [159] |
| P3HT ** | Diels-Alder ligation | A facile covalent strategy was developed to address the bundling issue of CNTs | [160] | |
| MWCNT | PEDOT:PSS | Electrochemical co-deposition | A facile and effective approach for electrode preparation was reported | [54] |
| Ternary system | Details | Refs. | ||
|---|---|---|---|---|
| CP | Carbon | Inorganic species | ||
| PANI | GN/CNT | Intercalation of CNT between GN sheets | [163] | |
| In situ deposition of PANI on GN/CNT paper | ||||
| Graphite felt/CNT | Electropolymerization of PANI in the presence of graphite felt | [164] | ||
| Electrophoretic immobilization of CNT on the hybrid | ||||
| GN/CNF * | Production of G/CNF by electrospinning | [165] | ||
| In situ polymerization of PANI | ||||
| GO | S | Layer-by-layer synthesis of PANI layer on GO-S composite with heating | [166] | |
| rGO | MnO2 | PANI vertically grown on GO sheet | [167] | |
| Reduction of GO and follow by the deposition of MnO2 onto PANI | ||||
| rGO/CNT | Combination of chemical foaming, thermal reduction, and KOH activation to prepare rGO | [168] | ||
| Interfacial polymerization | ||||
| N-doped rGO | NiFe2O4 | NiFe2O4 simultaneously grown with reduction and doping of GO | [169] | |
| In situ chemical polymerization of PANI | ||||
| CNT | Fe | Reduction of FeCl3 in the mixing solution of aniline and CNTs | [170] | |
| CNT | CoS1.097 | Hydrothermal synthesis of CNT/CoS1.097 | [171] | |
| In situ electropolymerization of PANI | ||||
| rGO | MnFe2O4 | Hydrothermal reaction to disperse MnFe2O4 well on the rGO surface | [172] | |
| In situ polymerization of PANI | ||||
| Vertical rGO | Pd | One-step electrodeposition of PANI and rGO | [173] | |
| Spontaneous redox reaction of PANI with Pd salt | ||||
| CNT | Ag | In situ chemical polymerization of PANI in the presence of AgNO3 and MWCNTs | [174] | |
| C | TiN | Sequential coating of C and PANI on the surface of TiN nanowire array | [175] | |
| C | S | In situ polymerization of aniline on the pore surface of mesoporous C which served as a reservoir for S | [176] | |
| PPy | rGO/CNT | Reduction of GO in the presence of CNT | [177] | |
| In situ chemical polymerization of PPy in the presence of rGO/CNT powders | ||||
| GN | S | In situ polymerization of PPy in the presence of GN | [178] | |
| Suspension mixture of PPy/GN with nano-S | ||||
| CNT | TiO2 | Chemical preparation of complex of TiO2/CNT | [179] | |
| In situ polymerization using MO as template | ||||
| PPy/PEDOT | CNT | Series of processes: high-temperature reflux technique, thermal compression, oxygen plasma etching, and electrochemical polymerization | [180] | |
| PT | Pd/TiO2 | Water-in-oil emulsion of TiO2 microspheres | [181] | |
| Loading Pd species and coating PT | ||||
| PEDOT:PSS | rGO | RuO2 | Mechanical stirring and sonochemical treatment of PEDOT:PSS in PSS-coated rGO solution | [182] |
| Chemical interaction between RuO2 and hybrid | ||||
| GO/CNT | Self-assembled interfacial coupling method | [123] | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.N.; Yoon, H. Recent Advances in Nanostructured Conducting Polymers: from Synthesis to Practical Applications. Polymers 2016, 8, 118. https://doi.org/10.3390/polym8040118
Nguyen DN, Yoon H. Recent Advances in Nanostructured Conducting Polymers: from Synthesis to Practical Applications. Polymers. 2016; 8(4):118. https://doi.org/10.3390/polym8040118
Chicago/Turabian StyleNguyen, Duong Nguyen, and Hyeonseok Yoon. 2016. "Recent Advances in Nanostructured Conducting Polymers: from Synthesis to Practical Applications" Polymers 8, no. 4: 118. https://doi.org/10.3390/polym8040118
APA StyleNguyen, D. N., & Yoon, H. (2016). Recent Advances in Nanostructured Conducting Polymers: from Synthesis to Practical Applications. Polymers, 8(4), 118. https://doi.org/10.3390/polym8040118