Crosslinked and Dyed Chitosan Fiber Presenting Enhanced Acid Resistance and Bioactivities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Crosslinking and Dyeing
2.2.1. Crosslinking Treatment
2.2.2. Adsorption and Dyeing Experiments
2.3. Measurements
2.3.1. Acid Resistance Test
2.3.2. FT-IR Analysis
2.3.3. Adsorption Measurements
2.3.4. Bioactivity Tests
3. Results and Discussion
3.1. Crosslinking of Chitosan Fiber
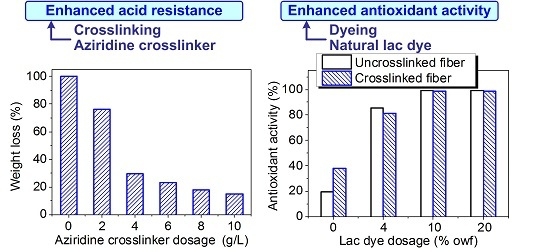
3.1.1. Crosslinking Conditions and Acid Resistance of Chitosan Fiber
3.1.2. Crosslinking Mechanism
3.2. Lac Dyeing of Chitosan Fiber
3.2.1. Equilibrium Adsorption Isotherms of Lac Dye
3.2.2. Building-up Properties of Lac Dye
3.3. Bioactivities of Chitosan Fiber
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Kitiyanant, Y.; Misra, R.D.K. Chitosan as a scaffold matrix in tissue engineering. Mater. Sci. Technol. 2008, 24, 1062–1075. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S. Wet-spinning and applications of functional fibers based on chitin and chitosan. Macromol. Symp. 2001, 168, 21–30. [Google Scholar] [CrossRef]
- Cimilli, S.; Nergis, B.U.; Candan, C.; Özdemir, M. A comparative study of some comfort-related properties of socks of different fiber types. Text. Res. J. 2010, 80, 948–957. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Maricato, E.; Cunha, A.; Nunes, A.; Silva, J.A.L.D.; Coimbra, M.A. Chitosan-caffeic acid-genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohyd. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Tang, R.-C. Crosslinking of chitosan fiber by a water-soluble diepoxy crosslinker for enhanced acid resistance and its impact on fiber structures and properties. React. Funct. Polym. 2016, 100, 116–122. [Google Scholar] [CrossRef]
- Ghosh, P.; Rameshbabu, A.P.; Das, D.; Francis, N.K.; Pawar, H.S.; Subramanian, B.; Pal, S.; Dhara, S. Covalent cross-links in polyampholytic chitosan fibers enhances boneregeneration in a rabbit model. Colloid. Surf. B 2015, 125, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Austero, M.S.; Donius, A.E.; Wegst, U.G.K.; Schauer, C.L. New crosslinkers for electrospun chitosan fiber mats. I. Chemical analysis. J. R. Soc. Interface 2012, 9, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Girase, B.; Shah, J.S.; Misra, R.D.K. Structure–process–property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 2011, 7, 3432–3445. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Shah, J.S.; Misra, R.D.K. Degradation mechanism and increased stability of chitosan-based hybrid scaffolds cross-linked with nanostructured carbon: Process–structure–functional property relationship. Polym. Degrad. Stabil. 2013, 98, 2331–2339. [Google Scholar] [CrossRef]
- Depan, D.; Misra, R.D.K. Processing–structure–functional property relationship in organic–inorganic nanostructured scaffolds for bone-tissue engineering: The response of preosteoblasts. J. Biomed. Mater. Res. A 2012, 100A, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Venkatsurya, P.K.C.; Girase, B.; Misra, R.D.K. Organic/inorganic hybrid network structure nanocomposite scaffolds based on grafted chitosan for tissue engineering. Acta Biomater. 2011, 7, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohyd. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Z.; Zhang, X.; Zhu, X.; Nie, J.; Ma, G. Crosslinked polyelectrolyte complex fiber membrane based on chitosan–sodium alginate by freeze-drying. RSC Adv. 2014, 4, 41551–41560. [Google Scholar] [CrossRef]
- Depan, D.; Kumar, A.P.; Singh, R.P. Cell proliferation and controlled drug release studies of nanohybrids based on chitosan-g-lactic acid and montmorillonite. Acta Biomater. 2009, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Pratheep Kumar, A.; Singh, R.P.; Misra, R.D.K. Stability of chitosan/montmorillonite nanohybrid toward enzymatic degradation on grafting with poly(lactic) acid. Mater. Sci. Technol. 2014, 30, 587–592. [Google Scholar] [CrossRef]
- Qin, C.Q.; Du, Y.M.; Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stabil. 2002, 76, 211–218. [Google Scholar] [CrossRef]
- Wei, Y.C.; Hudson, S.M.; Mayer, J.M.; Kaplan, D.L. The crosslinking of chitosan fibers. J. Polym. Sci. Part A 1991, 30, 2187–2193. [Google Scholar] [CrossRef]
- Knaul, J.Z.; Hudson, S.M.; Creber, K.A.M. Crosslinking of chitosan fibers with dialdehydes: Proposal of a new reaction mechanism. J. Polym. Sci. Part B 1999, 37, 1079–1094. [Google Scholar] [CrossRef]
- Yang, Q.; Dou, F.; Liang, B.; Shen, Q. Investigations of the effects of glyoxal cross-linking on the structure and properties of chitosan fiber. Carbohyd. Polym. 2005, 61, 393–398. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, J.-H. Fiber formation and physical properties of chitosan fiber crosslinked by epichlorohydrin in a wet spinning system: The effect of the concentration of the crosslinking agent epichlorohydrin. J. Appl. Polym. Sci. 2004, 92, 2054–2062. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Martínez, V.; Rubio, L.; Parra, J.L.; Coderch, L. Antioxidant cosmeto-textiles: Skin assessment. Eur. J. Pharm. Biopharm. 2013, 84, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L.C. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J.; Tang, R.-C. Adsorption and functional properties of natural lac dye on chitosan fiber. React. Funct. Polym. 2013, 73, 1559–1566. [Google Scholar] [CrossRef]
- Fras-Zemljič, L.; Kokol, V.; Čakara, D. Antimicrobial and antioxidant properties of chitosan-based viscose fibers enzymatically functionalized with flavonoids. Text. Res. J. 2011, 81, 1532–1540. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Piffaut, B.; Rondeau-Mouroc, C.; Girardin, M.; Jasniewski, J.; Scher, J.; Muniglia, L. Functionalization of chitosan by laccase-catalyzed oxidation of ferulic acid and ethyl ferulate under heterogeneous reaction conditions. Carbohyd. Polym. 2012, 87, 537–544. [Google Scholar] [CrossRef]
- Božič, M.; Štrancar, J.; Kokol, V. Laccase-initiated reaction between phenolic acids and chitosan. React. Funct. Polym. 2013, 73, 1377–1383. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Guebitz, G.M.; Kokol, V. Antimicrobial and antioxidant properties of chitosan enzymatically functionalized with flavonoids. Process Biochem. 2009, 44, 749–756. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Arguelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohyd. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Tasselli, F.; Mirmohseni, A.; Seyed Dorraji, M.S.; Figoli, A. Mechanical, swelling and adsorptive properties of dry–wet spun chitosan hollow fibers crosslinked with glutaraldehyde. React. Funct. Polym. 2013, 73, 218–223. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Huang, K.-L.; Lee, J.-F. Preparation and morphology studies of nano zinc oxide obtained using native and modified chitosans. Materials 2013, 6, 4198–4212. [Google Scholar] [CrossRef]
- Valderruten, N.E.; Valverde, J.D.; Zuluaga, F.; Ruiz-Durántez, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014, 84, 21–28. [Google Scholar] [CrossRef]
- Azlan, K.; Wan Saime, W.N.; Lai Ken, L. Chitosan and chemically modified chitosan beads for acid dyes sorption. J. Environ. Sci. 2009, 21, 296–302. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Chuang, G.-S. Competitive adsorption of dye metanil yellow and RB15 in acid solutions on chemically cross-linked chitosan beads. Chemosphere 2006, 62, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Equilibrium studies for acid dye adsorption onto chitosan. Langmuir 2003, 19, 7888–7894. [Google Scholar] [CrossRef]
- Zhou, Z.; Lin, S.; Yue, T.; Lee, T.-C. Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J. Food Eng. 2014, 126, 133–141. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Adsorption of acid dyes on chitosan-equilibrium isotherm analyses. Process Biochem. 2004, 39, 693–702. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuziere, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q.; Gadre, A.P.; Yi, H.; Kastantin, M.J.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface. Langmuir 2002, 18, 8620–8625. [Google Scholar] [CrossRef]
- Ketmaro, P.; Muangsiri, W.; Werawatganone, P. UV spectroscopic characterization and stabilities of natural colorants from roselle calyx, lac resin and gardenia fruit. J. Health Res. 2010, 24, 7–13. [Google Scholar]
- Tang, R.-C.; Tang, H.; Yang, C. Adsorption isotherms and mordant dyeing properties of tea polyphenols on wool, silk and nylon. Ind. Eng. Chem. Res. 2010, 49, 8894–8901. [Google Scholar] [CrossRef]
- Kremer, D.; Kosalec, I.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Zovko Končić, M. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark. Food Chem. 2012, 131, 1174–1180. [Google Scholar] [CrossRef]







| Model | ND (%) | |
|---|---|---|
| Uncrosslinked | Crosslinked | |
| Langmuir | 22.65 | 24.43 |
| Freundlich | 10.21 | 11.01 |
| Langmuir + Nernst | 6.60 | 6.12 |
| Fiber | KL (L/mg) | S (mg/g) | KP (L/mg) |
|---|---|---|---|
| Uncrosslinked | 0.0245 | 159.7 | 0.130 |
| Crosslinked | 0.0327 | 149.6 | 0.110 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-Q.; Tang, R.-C. Crosslinked and Dyed Chitosan Fiber Presenting Enhanced Acid Resistance and Bioactivities. Polymers 2016, 8, 119. https://doi.org/10.3390/polym8040119
Li X-Q, Tang R-C. Crosslinked and Dyed Chitosan Fiber Presenting Enhanced Acid Resistance and Bioactivities. Polymers. 2016; 8(4):119. https://doi.org/10.3390/polym8040119
Chicago/Turabian StyleLi, Xiao-Qiong, and Ren-Cheng Tang. 2016. "Crosslinked and Dyed Chitosan Fiber Presenting Enhanced Acid Resistance and Bioactivities" Polymers 8, no. 4: 119. https://doi.org/10.3390/polym8040119
APA StyleLi, X. -Q., & Tang, R. -C. (2016). Crosslinked and Dyed Chitosan Fiber Presenting Enhanced Acid Resistance and Bioactivities. Polymers, 8(4), 119. https://doi.org/10.3390/polym8040119